
ORIGINAL
Community
pharmacy-based interventions with Valeriana officinalis or Passiflora
incarnata together with sleep hygiene education improve climacteric
symptoms and sleep problems in menopause
Las
intervenciones farmacéuticas con Valeriana officinalis o Passiflora incarnata
junto con la educación en la higiene del sueño mejoran los síntomas climatéricos
y los problemas del sueño en la menopausia
Elena
Marcos1, Irene Iglesias1, Miguel Vazquez-Velasco1,
Juana Benedi1
1Department
of Pharmacology, Pharmacognosy and Botanical, Faculty of Pharmacy, Universidad
Complutense de Madrid, Plaza Ramon y Cajal s/n, Ciudad Universitaria, 28040,
Madrid, Spain.
*
Corresponding Author.
|

This work
is licensed under a Creative
Commons
Attribution-NonCommercial-ShareAlike 4.0 International
License
La
revista no cobra tasas por el envío de trabajos,
|
|
Abstract
Introduction.
Physiological and endocrine changes occur during menopause that can negatively
affect the sleep-wake cycle and contribute to objective and subjective sleep
problems.
Objective. To assess
the effectiveness of a pharmaceutic intervention with two different
complementary treatments and sleep hygiene education on climacteric symptoms
and sleep domains in menopausal women with sleep disturbance.
Material and methods. A sample of 109 women (45-64 years) participated in a
3-month randomized study, 35 received sleep hygiene instructions (SHI), 36
received capsules containing Passiflora incarnata 3 times a day plus SHI
(PI), and 38 received capsules containing Valerian officinalis 3 times a
day plus SHI (VO). Participants were evaluated by a) the Menopause Quality of
Life (MENQOL) instrument, b) Pittsburgh Sleep Quality Index (PSQI), c) Insomnia
Severity Index, d) Epworth Sleepiness Scale, and e) Mental component of SF-12 health survey.
Results. MENQOL
scores were similar at baseline in the three groups but were reduced (improved
vasomotor domain and physical subscale) at the end of the study in the VO group
when compared with PI and SHI counterparts (both, p<0.05). The SF-12 mental
function showed improvement in the VO group (p<0.05). Global PSQI score was
significantly improved by PI and VO treatments at the end of treatment (p=0.046
and p=0.034, respectively). VO group was more effective than PI in alleviating
mild insomnia. Change in vasomotor symptoms positively and significantly
correlated with changes in all items of PSQI components, except for sleep
duration and the association was strongest with sleep latency. Most
participants evaluated the pharmaceutical and educational interventions
provided as satisfactory.
Conclusions. The Valerian officinalis was the preferable treatment
for the climateric symptoms and sleep difficulties associated with menopause.
This study provided evidence that community pharmacists can play a crucial role
in referring menopausal women with symptoms of insomnia to potential medicinal
plants therapy and sleep hygiene instructions.
Keywords
Valerian officinalis;
Passiflora incarnate; community pharmacy; climacteric symptoms; sleep
disturbance; menopause
Resumen
Introducción. Durante
la menopausia se producen cambios fisiológicos y endocrinos que pueden afectar negativamente
al ciclo sueño-vigilia y contribuir a problemas objetivos y subjetivos del
sueño.
Objetivos. Evaluar la
efectividad de la intervención farmacéutica con dos tratamientos
complementarios diferentes y educación en la higiene del sueño sobre los
síntomas climatéricos y los dominios del sueño en mujeres menopáusicas con
trastornos del sueño.
Métodos. Una
muestra de 109 mujeres (45-64 años) participaron en un estudio aleatorizado de
3 meses, 35 recibieron instrucciones de higiene del sueño (SHI), 36 recibieron
cápsulas que contenían Passiflora incarnata 3 veces al día más SHI (PI),
y 38 recibieron cápsulas que contenían Valerian officinalis 3 veces al
día más SHI (VO). Las participantes completaron los cuestionarios de a) calidad
de vida especifica de la menopausia (MENQOL), b) índice de calidad del sueño de
Pittsburgh (PSQI), c) índice de gravedad del insomnio, d) escala de somnolencia
de Epworth y e) dimensión salud mental del cuestionario de salud SF-12.
Resultados. Las
puntuaciones del cuestionario de calidad de vida específico de la menopausia
fueron similares en todos los grupos al inicio del estudio y se redujeron
(dominio vasomotor y subescala física) al final del estudio en el grupo VO en
comparación con PI y SHI (ambos, p <0.05). La función mental SF-12 mostró
una mejoría en las mujeres del grupo VO (p <0.05). La puntuación global de
PSQI mejoró significativamente con PI y VO al final del tratamiento (p = 0.046
y p = 0.034, respectivamente). El grupo VO fue más efectivo que PI para aliviar
el insomnio leve. El cambio en los síntomas vasomotores mostró correlaciones
significativas positivas con todos los ítems en los componentes del PSQI,
excepto en la duración del sueño. La asociación fue más mayor con la latencia
del sueño. La mayoría de las participantes evaluaron las intervenciones
farmacéuticas y educativas prestadas como satisfactorias.
Conclusiones. La Valeriana officinalis asociada a la higiene del
sueño fue el tratamiento preferible para los síntomas climáticos y las
dificultades de sueño en la menopausia. Este estudio proporcionó evidencia de
que los farmacéuticos comunitarios pueden desempeñar un papel importante
derivando a las mujeres menopáusicas con síntomas de insomnio a la terapia
potencial de plantas medicinales e higiene del sueño.
Palabras clave
Valerian officinalis; Passiflora incarnate; farmacia
comunitaria; síntomas climatéricos; alteraciones
del sueño; menopausia
Introduction
The menopausal period is
characterized by a number of physiological and endocrine changes that may
adversely affect the sleep-wake cycle, and contribute to both objective and
subjective problems in sleep(1). Approximately 90% of women
experience menopausal symptoms, including sleep disorders(1), which
may be related to the vasomotor domain as suggested by Lampio et al.(2),
but still there is no extended consensus supporting this view.
Polo-Kantola(3)
reported that 25% of women aged 50 to 64 years have sleep problems, and 15% of
them state that severe sleep disturbance has a substantial effect on their
quality of life. Thus, the presence of menopausal symptoms increases health
care utilization and costs, as well as sick leave days(4). Clinical
guidelines recommend to start treating these symptoms, with non-pharmacological
therapy(5), including medicinal plants, which are considered safe
and available for most consumers(6).
Different studies indicate
that phytoestrogens are particularly effective on vasomotor and psychosomatic
symptoms of menopause(7). In addition, there have been reported
improvements on sleep and memory symptoms in menopausal women using herbal
medicines (8). Among them, Valerian officinalis and Passiflora
incarnata has been reported to improve vasomotor symptoms, insomnia, and
depression(8-10). Commission E monographs recommend valerian root as
a safe and effective treatment for the vasomotor symptoms of menopause,
although little evidence support the use of valerian in treatment of hot
flashes(11,12). On the other hand, Passiflora incarnata has
been recommended as a therapy for insomnia(11-13), anxiety(10-13)
and hot flashes but not for the vasomotor symptoms(11).
Community pharmacists are in
a suitable position to provide ongoing follow-up related to a range of health
problems(14). Several pharmacy-based studies evaluating the control
of insomnia have been performed(15,16), but to the best of our
knowledge, no interventions have been carried out to provide women information
about medicinal plants and complementary therapies for menopause and sleep
problems, which allow them to make an informed choice about how to relieve
their symptoms.
We hypothesize that
medicinal plants combined with sleep hygiene instructions could have a synergic
effect on climacteric symptoms, sleep problems and women’ s health. Thus, the
aim of this study was to assess in a cohort of menopausal women with sleep
disturbance attending to the Community Pharmacy Services, the effectiveness of
a pharmacist-led intervention with medicinal plants combined with sleep hygiene
instructions on sleep quality and climateric symptoms outcomes compared with
sleep hygiene instructions alone over a 3 month period.
Material and methods
This is a community-based
intervention, prospective, quasi-experimental with pre-test and post-test
groups. Seven community pharmacies in Spain (three in Madrid, two in Toledo and
two in Guadalajara) were invited to participate and express interest to join
the study. The study period was January 2017 to September 2018.
Previous studies, based on
the experience and suggestion of pharmacists who have participated in community
research work, establish between 10 and 20 the reasonable number of volunteers
by each pharmacist(17). A total of 132 perimenopause women between
45-65 years with sleep problems, who had not received prior hypnotic or
alternative treatments and who voluntarily requested sleep aid, were recruited
consecutively in the participating pharmacies. Inclusion criteria were as
described in Table 1. All woman with a history of hormone replacement therapy
for the management of menopausal symptoms, were suffering from any kind of
heart disease, hypertension and/or diabetes, if they were currently undergoing
chemotherapy or had received any previous treatment for insomnia, were excluded
from analysis. Finally, 109 women completed the study.
Table
1. Inclusion criteria
|
a) Menopausal women, aged 45 to 64 years
|
|
b) Subjective complaint of difficulties initiating (sleep
latency >30 min) and/or maintaining sleep (time awake after sleep onset
>30 min) for a minimum of 2 nights and a maximum of 5 nights per week and
for at least a 1-month duration
|
|
c) General good health without evidence of clinical disease.
|
Randomization was performed
at the participating level in each pharmacy. All groups received sleep hygiene
instructions (SHI) for improving insomnia (Table 2) based in studies on sleep
disorders treatment in the elderly and non-pharmacological treatment of chronic
insomnia (18). The importance of the daily application of those
instructions for three months was emphasized.
Table
2. Sleep hygiene instructions
|
Wake up at the same time and go to bed at the same time,
every day.
|
|
Avoid naps, except for a brief 10- to 15-minute nap no
later than 4 pm.
|
|
Take regular mild-to-moderate exercise terminating ≥4 hours
before bedtime.
|
|
Do not smoke to get yourself back to sleep.
|
|
Limit caffeine use to ≤3 cups per day and not after 4 pm.
|
|
Limit alcohol consumption to light-to-moderate quantities.
|
|
Do not eat or drink heavily 3 hours before bedtime. A light
bedtime snack may be helpful.
|
|
Use the bedroom only for sleep; do not work or do other
activities that lead to prolonged arousal.
|
|
Do not stay in bed for more than 30 minutes without
sleeping.
|
|
Get up and go to another room, try to engage in a relaxing
activity (music, reading) and concentrate on pleasant feelings.
|
|
Use a bedtime ritual or read before lights out – this may
be helpful if it is not work-related.
|
Adapted
from Joshi(18)
The medicinal plant
treatment groups received medicinal plant therapy: Group PI received capsules
containing 300 mg of Passiflora incarnata 3 times a day for three months
while group VO received capsules containing 350 mg of Valerian officinalis
root 3 times a day for three months. Semi-structured face-to face interviews
with participants and data collection were recorded by the researcher pharmacist
in charge of the Pharmaceutical Care study, pre- and post-test, in order to
guarantee rigor and homogeneity, for all participants.
Ethical principles were
taken into consideration at all stages of the study. Ethical approval was
obtained from the School of Pharmacy Ethics Committee, the Complutense
University of Madrid (Reference number: PR016/05). Women were informed about
the purpose and the course of the study and that they were free to withdraw at
any stage. Women were assured about the confidentiality of the data and the
absence of any constraints to participate.
Study Parameters
At baseline, the research
obtained data pertaining to participants’ socio-demographic and clinical
characteristics. We evaluated menopausal-specific symptoms using the Menopause
Quality of Life instrument (MENQOL), divided into four scales, assessing
vasomotor (three items), psychosocial (seven items), physical (sixteen items)
and sexual (three items) domains, with the score on each ranging from 1 (not
experiencing a symptom) to 8 (extremely bothered). Thus, high scores in MENQOL
subscales indicate low quality of life(19). Different questionnaires
were used for insomnia: a) The Pittsburgh Sleep Quality Index (PSQI) is a
self-rated questionnaire assessing sleep quality and disturbances over a
1-month time interval, with higher scores indicating worse sleep quality(20);
b) Insomnia Severity Index (ISI). The ISI is a brief, reliable, validated
self-reporting instrument that yields a quantitative index of perceived insomnia
severity, where higher scores reveal more severe insomnia (0–7, absence of
insomnia; 8–14, mild insomnia; 15–21, moderate insomnia; 22–28, severe
insomnia)(21); c) Epworth Sleepiness Scale (ESS). The ESS was used
to measure the severity of daytime sleepiness. Respondents rated eight items
regarding the likelihood of dozing in sedentary situations on a scale from 0
(never) to 3 (high chance)(22); d) The Mental Component Summary
scale (MCS) from the 12-item short-form (SF-12) was used to assess the emotional
role, vitality, social functioning, and mental health in menopausal women
before and after treatment. Higher scores indicate a greater quality of life(23).
A satisfaction survey was performed based on the Treatment Satisfaction
Questionnaire for Medication (TSQM version 1.4) to find out the participant´s
acceptance grade of treatment(24). Participants were instructed not
to respond to the questions in the side effects dimension if they were not
suffering from side effects. Pharmacists conducted follow-ups with included
participants during the study period (start, one, three months) but only the
first and latest was considered in this study to assess for resolution of
symptoms, adherence to therapy and any adverse events.
Statistical analysis
The results were presented
as mean and standard deviation (SD) for quantitative variables and percentages
for categorical variables. Chi-square test and one way ANOVA followed by Tukey post
hoc analysis were used to examine differences in the demographic variables
among the three groups. Changes were evaluated as rate of change considering
the following equation:
Rate of change (%) = 100*
(Postintervention - Preintervention)/Preintervention
The rate of change in
measured outcomes of climacteric symptoms and sleep problems were evaluated by
univariate repeated measurement test considering the treatment groups (SHI, PI,
and VO) followed by the GLM post hoc analysis and the significance of
the intervention in each group evaluated. Spearman correlations were applied to
assess the association between changes in menopausal symptom scores and mental
component score (SF-12 MCS) with change in PSQI scores or other related
parameters. A correlation coefficient ≥0.75 was considered good to excellent;
0.50–0.75, moderate to good; 0.25–0.50, fair; and 0.00–0.25, little to no
relationship(25). Satisfaction of the two treatment modalities and
SHI group using TSQM scale were compared using the Wilcoxon test. The p-values
were considered significant at p <0.05.
Results
All analyses were limited to
women who completed all assessments (n=109)(26). The demographic
characteristics of the study participants are shown in Table 3. Differences
among the three groups were not significant.
Table
3. Demographic and clinical characteristics of study
participants
|
Variable
|
SHI
(n=35)
|
PI
(n=36)
|
VO
(n=38)
|
Significance*
|
|
Age (years)
Mean (SD)
|
59.6 (5.1)
|
58.2 (5.7)
|
59.1 (6.1)
|
0.75
|
|
Educational level (%)
Elementary (<10 years)
High school (10-15 years)
College graduate (>15 years)
|
4.9
43.8
51.3
|
4.1
42.1
50.2
|
4.6
44.7
52.5
|
0.96
|
|
Marriage status (%)
Single
Married
|
27.3
72.7
|
30.3
69.7
|
22.2
77.8
|
0.26
|
|
Occupation status (%)
Employed
Housewife
|
73.1
26.9
|
74.9
25.1
|
78.7
21.3
|
0.54
|
|
BMI (kg/m2)
Mean (SD)
|
27.3 (2.8)
|
29.1 (4.5)
|
26.9 (3.1)
|
0.67
|
|
Smoking (%)
No
Si
|
85.9
14.3
|
87.5
13.3
|
83.7
16.3
|
0.56
|
|
Alcohol consumption (%)
No
Yes (≥3 times/week)
|
89.1
10.9
|
87.8
12.1
|
90.7
9.3
|
0.33
|
|
Caffeinated beverages (%)
None
>3 serving per day
|
76.3
23.7
|
73.9
26.1
|
73.2
28.8
|
0.41
|
BMI,
body mass index; SHI, sleep hygiene instructions; PI, capsules containing 300
mg of Passiflora incarnata; VO, capsules containing 350 mg of Valerian
officinalis; SD standard deviation; *Chi-square test or one-way ANOVA test
were used to examine differences in percentage or absolute value, respectively,
for demographic variables among groups.
Quality of life evaluation
Table 4 shows that the rate of changes of vasomotor and physical
domains of MENQOL and SF-12 MCS score differ between groups according to GLM
analysis (p<0.05). The study results revealed that vasomotor and physical
subscale of the MENQOL score decreased (all p<0.05) follow after the intervention with VO over time. Following the VO
(p<0.01) and PI (p<0.05) treatments, the SF-12 MCS score increases. The
rate of changes of vasomotor and physical domains differed between the SHI and
PI group versus VO group. The rate of changes of SF-12 MCS score differed
between PI and VO group versus SHI group (p<0.05).
Subjective Sleep Quality
Table 5 shows that the rate
of changes of percentage of menopausal woman with poor sleep habit differ
between groups according to Chi-square analysis (p<0.01). It is observed
after intervention with PI (p<0.05) and VO (p<0.01) a significant
reduction in the prevalence of women with sleep problems. The rate of changes
of poor sleepers (%) differed (p<0.05) between the PI and VO treatments
versus SHI group.
Table 5 also shows rates
of change for global PSQI and PSQI subcomponents differ significantly among
treatments in women according to GLM analysis (at least p<0.05). All
variables but sleep disturbances were significantly affected. Both the PI and
VO groups had significantly reduction in most of PSQI subscale scores at post-treatment.
Global PSQI, sleep latency and subjective sleep quality decrease in VO and PI
groups (at least p<0.05) while duration of sleep increase in VO group. The
rate of changes of subjective sleep quality, sleep latency and duration of
sleep differed (p<0.05) in the VO group versus PI and SHI groups.
The rates of change for ESS
and ISI scores differ between treatments in women with sleep problems according
to GLM analysis (p<0.05). A significant decrease in ESS and ISI scores was
observed in the PI group (p<0.05) and VO group (p<0.01). At baseline
menopausal women in all groups had a mean ISI total score of 13.5, which
corresponds to “mild insomnia” but at the end of the study mean ISI score of
the VO group changed from 13.7 (3.4) at baseline to 7.0 (2.8) and in the PI
group, it altered from 14.3 (2.5) to 9.4 (3.1) after 3 months (p<0.01,
p<0.05, respectively). The rate of changes of ISI score differed (p<0.05)
between the VO group versus PI and SHI groups.
Figure 1 shows that after 3 months, the percentage of menopausal
women who declare moderate insomnia reduction from baseline were larger in the
VO group (25.4 vs 5.2%) than in the group that received PI (30.3 vs 17.5%). In
addition, the VO treatment alleviated mild insomnia (70.vs 30.4%) more effectively
than the PI counterpart (67.1 vs 49.8, respectively). Evaluation of the
participants after the treatment showed that in the VO and PI groups 63.4 % and
31.4% respectively, of the menopausal women did not report any degree of
insomnia whereas 18.1% in the SHI group (Figure 1).
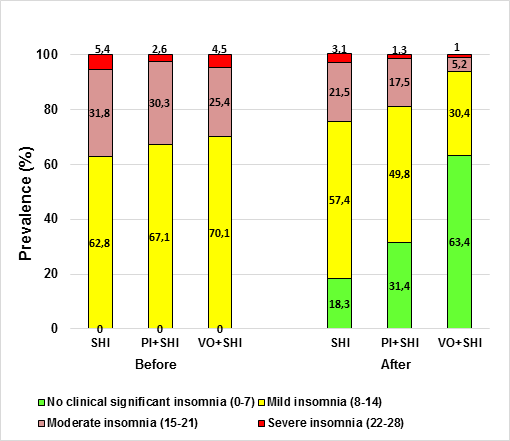
Figure
1. Percentage of menopausal women responders based on ISI
scoring in control. PI (capsules containing 300 mg of Passiflora incarnate)
and VO (capsules containing 350 mg of Valerian officinalis) groups,
before and after treatment; SHI, sleep hygiene instructions.
The increase in
“non-clinical significant insomnia” was mostly due to a reduction in the
percentages of participants in the “severe insomnia” and “moderate insomnia”
categories relative to baseline.
Table 6 shows the correlations of the average change in MENQOL
(vasomotor and physical domains), MCS (SF-12) and PSQI scores with other
parameter of sleep. Average change in vasomotor symptoms showed significant
positive correlations with all items on the average changes the PSQI components
except to duration of sleep, and the association was strongest with sleep
latency (r=0.224; p=0.009). Also, there were significant and positive
correlation between the average changes in the vasomotor symptoms, and the
average changes in daytime sleepiness as assessed by the ESS (r=0.178;
p=0.021). All average changes in PSQI items except the subjetive sleep quality
were significantly associated with the average change in physical symptoms.
Duration of sleep was significantly correlated with physical symptoms (r=0.127;
p=0.024). The average changes insomnia severity as measured by ISI did not
correlated with average changes in vasomotor and physical symptoms. Average
change MCS (SF-12) was negatively and significantly correlated with average
change in all PSQI scores (p<0.05) and average changes in ISI. ISI score was
most highly correlated with mental SF-12 (r=-0.265; p<0.001).
Table
6. Spearman correlations between the average changes of
vasomotor and physical symptoms and SF-12 (MCS) mental component score and the
average changes of PSQI measures of Insomnia or related symptom severity
|
PSQI measure
|
Vasomotor
symptoms
r (p)
|
Physical symptoms
r (p)
|
SF-12 MCS
r (p)
|
|
Subjetive sleep quality
Sleep latency
Duration of sleep
|
0.123 (0.003)
0.224 (0.009)
0.087 (0.056)
|
0.082 (0.245)
0.075 (0.011)
0.127 (0.024)
|
-0.171(0.041)
-0.192 (<0.001)
-0.120 (0.024)
|
|
EES
|
0.178 (0.021)
|
0.109 (0.034)
|
-0.063 (0.63)
|
|
ISI
|
0.104 (0.89)
|
0.033 (0.44)
|
-0.265 (<0.001)
|
EES,
Epworth Sleepiness Scale; ISI, Insomnia Severity Index; SF-12, 12-Item
Short-Form Health Survey; MCS mean mental health related quality of life SF-12.
Global Satisfaction and tolerability
Treatments Users’
satisfaction was calculated using the TSQM scoring algorithm. In the perception
of efficacy, the average satisfaction of menopausal women was significantly
higher with Valerian officinalis vs. with Passiflora incarnate (17.1/21
vs 14.7/21, respectively; p=0.03). In the second block of questions, 2 women
described adverse effects (only with Valerian officinalis). These women
detailed dyspepsia. Regarding to convenience, the assessment was 16.4/21 with Valerian
officinalis vs. 16.1/21 with Passiflora incarnata (p=0.564). The
global satisfaction level was 13.1/17 with Valerian officinalis and
12.8/17 with Passiflora incarnata (p=0.51). There were no statistically
significant differences in the score obtained for these last two sections.
Participants receiving SHI alone presented lower score values (effectiveness
10.1/21; global satisfaction 11.4/17) except in side-effects (not applicable)
and convenience score (17.9/21).
Discussion
Present
study evaluates the effectiveness of a community pharmacy medication support
service for menopausal women with common insomnia. Although positive outcomes
have been reported from clinical studies on herbal medicines, to the best of
our knowledge this is the first report comparing capsules of Passiflora incarnata
with capsules Valerian officinalis for relieving climateric symptoms in
menopausal women with sleep problems in the frame of a community pharmacy
intervention. In addition, the study demonstrates an improvement in the mental
quality of life of menopausal women using the medicinal plants suggested with
respect to the use of hygiene sleep techniques alone.
The results
of our study showed that daily consumption of 350 mg of Valerian officinalis
root 3 times a day for three months, in addition to SHI, significantly reduced
the vasomotor and physical symptoms of menopausal women in comparison to only
SHI. The reductions of climateric symptoms were modest, but similar in size to
that found in other studies of phytoestrogen supplements(27, 28).
Mirabia and Mojab(29) suggest that the reduction in severity and
frequency of hot flashes is merely due to the presence of phytoestrogens in
valerian, which can be administered to women suffering from hot flashes in a
simple and non-invasive manner. Furthermore, our study demonstrated the ability
of Valerian officinalis supplement to also benefit physical symptoms.
The changes in sleep quality correlated with changes in vasomotor and physical
symptoms, in line with other studies(10). In addition, a more
positive change (greater improvement in mental health) was associated with
lower somnolence values and greater subjective sleep quality. In particular,
physical symptoms showed the strongest association with sleep quality. In
comparison to Passiflora incarnata, we observed that Valerian
officinalis produced a higher benefit on commonly coexisting climacteric
symptoms for treatment of sleep disturbances. In contrast to our findings, Passiflora
incarnata treatment has been associated with significant improvements in
precocious menopause symptoms(10). However, no clear consensus is
able on bibliography(10,30). The study criteria allowed us to select
a population sample in which the onset of menopausal symptoms had preceded the
development of insomnia, and no other reasons for the occurrence of co-morbid
insomnia were detected. Treatment of insomnia with Valerian officinalis
resulted in reduction of awakenings due to nocturnal hot flushes, with a
positive impact on sleep quality and on the subsequent daytime function(29,31).
It could de hypothesized that the improvement in sleep duration with Valerian
officinalis resulted in decreased awareness of the occurrence/severity of
nocturnal hot flushes or increased threshold for awakenings caused by vasomotor
symptoms. In addition, a longer restful night could have had a positive effect
on daytime function and well-being and possibly influenced participants’
ability to tolerate diurnal vasomotor symptoms, as noted by improvement in
vasomotor sub-scores of the MENQOL questionnaire.
The proportion of poor
sleepers found at the beginning of our study (59.3%) was similar to that
reported by others(32,33), confirming the high prevalence of poor
sleeper among woman with climacteric symptoms. Present results demonstrated a
decline of ISI, ESS and PSQI scores in the frame of both medicinal plant
treatment groups. However, oral administration of Valerian officinalis
and Passiflora incarnata significantly but differentially improved the
subjective perception of sleep quality by PSQI. Thus, Valerian officinalis
administration induced greater effect on sleep latency, and duration than did
either Passiflora incarnata or the SHI protocol. A previous study
demonstrated that sleep disorders could impact the daytime functioning and
quality of life(33), which was confirmed by improved daytime
dysfunction after treatment with Valerian officinalis. In the VO group, the mean ISI scores at 3 month were reduced
by approximately 6-7 points as compared to its preintervention value. In the PI
group, the reduction was approximately 4 points; however, this decrease was
slightly smaller than the recommended reduction of 6 points for representing a
clinically meaningful improvement in individuals with primary insomnia(21).
Valerian officinalis was more effective than Passiflora incarnata
and the SHI in alleviating mild and moderate insomnia. The increase in
“non-clinical significant insomnia” was mostly due to a reduction in the
percentages of participants in the “severe insomnia” and “moderate insomnia”
categories relative to baseline. In addition, more than double Valerian
officinalis-treated women reported better sleep habits compared with those
of the SHI group. Although in SHI has been found to exert positive impact on
menopausal women life quality(34), present data suggest that SHI per
se was not sufficient to induce significant changes in this postmenopausal
population, and could be partially explained due to difficulties in producing
and maintaining significant lifestyle changes, especially in adult or elder people.
On the other hand, other studies assessing the impact of valerian on life
quality of postmenopausal women reported a lack of change in response after
1-year treatment(35), suggesting that plants treatment without SHI
were also not sufficient. Hence, it seems that the combination of SHI together
with medicinal plants treatment could have a synergic effect allowing
improvements in life quality(36) and climacteric symptoms(37).
As discussed, participants of our study were required in addition the the plant
treatment to follow SHI daily during the 3-month treatment, which may have
contributed to the improvements in sleep quality observed in both treatment
groups.
In addition, study results
clearly suggest that not all the medicinal plants labelled as “menopausal
treatments” affected the different symptoms equally. Thus, the importance of
the pharmacist assessment identifying the particular symptoms and choosing the
most adequate plant for each person was capital in both interventions.
When comparing satisfaction
of participants with insomnia and climacteric symptoms, a high satisfaction
rate for the capsules of VO, followed by the capsules of PI was observed. The
women of SHI group showed less satisfaction. These results are well in line
with those of several studies and may be attributed to the efficacy and
favorable risk-benefit profile(38). Perceived convenience is also an
important component of treatment satisfaction(23). Medicinal plants
play a central role not only as traditional medicines but also as trade
commodities, meeting the demand of menopausal woman with insomnia.
In line with other consumer
satisfaction surveys(19), this pharmacy based intervention was well
received by menopausal women. Nevertheless, the present study has several
limitations: 1) The intervention was applied by a limited number of pharmacies;
2) Multiple follow-up points and longer treatment would strengthen our results;
3) Absence of compliance monitoring; 4) Although we instructed the participants
to avoid drinking caffeine and alcohol, there was no way to ascertain their
compliance.
Conclusions
Valerian officinalis appear as the preferable treatment for the climateric
symptoms and sleep difficulties associated with menopause. Both
Valerian officinalis and Passiflora incarnata togheter with SHI ameliorated
several sleep associated symptoms, while sleep hygiene instructions perse was
unable to change women lifestyles enough to alleviate significantly those
symptoms. This study provided evidence that community pharmacists can play a
crucial role when addressing menopausal women with insomnia symptoms to the
most fitting complementary treatment with a combination of medicinal plants and
sleep hygiene instructions.
Acknowledgements
We thank the pharmacists
that intervened in the study for their support and willingness in recruiting
patients for this intervention. We also thank all volunteers for their time and
participation in the study.
Disclosure Statement
The authors have no
conflicts of interest to declare.
Author Contributions
Study concept and design:
Juana Benedi; supervision and writing of manuscript: Miguel Vazquez and Juana
Benedi; data acquisition, analysis and interpretation: Elena Marcos and Irene
Iglesias; statistical analysis: Elena Marcos. We confirm that all listed
authors meet the authorship criteria, and all authors agree with the content of
the manuscript.
Referencias
1.
Lee
J, Han Y, Cho HH, Kim MR. Sleep disorders and
menopause. J Menopausal Med. 2019; 25(2): 83-87.
2.
Lampio L, Polo-Kantola P, Polo O, Kauko T,
Aittokallio J, Saaresranta T. Sleep in midlife women: effects of menopause,
vasomotor symptoms, and depressive symptoms. Menopause 2014; 21(11): 1217-1224.
3.
Polo-Kantola P. Sleep problems in midlife and
beyond. Maturitas 2011; 68 (3):224-232.
4.
de Villiers TJ, Hall JE, Pinkerton JV, Perez
SC, Rees M, Yang C, et al. Revised global consensus
statement on menopausal hormone therapy. Maturitas 2016; 91: 153-155.
5.
Urru SA, Pasina L, Minghetti P, Giua C. Role
of community pharmacists in the detection of potentially inappropriate
benzodiazepines prescriptions for insomnia. Int J Clin Pharm 2015; 37(6):
1004-1008.
6.
Akram M, Daniyal M, Munir N, Mohiuddin E,
Sultana S. Medicinal plants combating against insomnia: A green anti-Insomnia
approach. J. Nerv Ment Dis 2019; 207(11): 927-935.
7. MacLennan
A, Lester S, Moore V. Oral oestrogen replacement therapy versus placebo for hot
flushes. Cochrane Database Syst Rev 2001; (1): CD002978.
8.
Kargozar R, Azizi H, Salari R. A review of
effective herbal medicines in controlling menopausal symptoms. Electron
Physician 2017; 9(11): 5826-5833.
9.
Taavoni S, Ekbatani N, Kashaniyan M, Haghani
H. Effect of valerian on sleep quality in postmenopausal women: a randomized
placebo-controlled clinical trial. Menopause 2011; 18(9): 951-955.
10.
Kim M, Lim H, Lee H, Kim T. Role
identification of Passiflora incarnata Linnaeus: A mini review. J
Menopausal Med 2017; 23 (3):156-159.
11.
Weil V, Cirigliano M, Battistini P.
Perspectives in complementary medicine: Herbal treatments for symptoms of
menopause clinical. Hospital Physician 2000; 36(11): 35-44.
12.
Lee J, Jung H-Y, Lee SI, Choi JH, Kim S-G.
Effects of Passiflora incarnata Linnaeus on polysomnographic sleep
parameters in subjects with insomnia disorder: a double-blind randomized
placebo-controlled study. Int Clin Psychopharmacol 2020; 35(1): 29-35.
13.
Ayala F, Mexicano G. Effect of a medicinal
plant (Passiflora incarnata L) on sleep. Sleep Sci 2017; 10(3): 96-100.
14. Merritt
S, Gyllenhaal C, Peterson S, Block K, Gochenour T. Herbal remedies: efficacy in
controlling sleepiness and promoting sleep. Nurse Pract Forum 2000; 11(2):
87-100.
15.
Kashyap KC, Nissen LM, Smith S, Kyle G.
Management of over-the-counter insomnia complaints in Australian community
pharmacies: a standardized patient study. Int J Pharm Pract 2014;
22(2):125-134.
16.
Driot D, Ouhayoun S, Perinelli F,
Grezy-Chabardes C, Birebent J, Bismuth M, et al. Non-drug and drug alternatives
to benzodiazepines for insomnia in primary care: Study among GPs and pharmacies
in a Southwest region of France. Therapie 2019; 74 (5): 537-546.
17.
Simpson S, Johnson J, Biggs C. Practice –
Based research: lessons from Community Pharmacist Participants. Pharmacotherapy
2001; 21: 731-739.
18.
Joshi S. Nonpharmacologic Therapy for
Insomnia in the Elderly. Clin Geriatr Med 2008; 24: 107–119.
19. Hilditch
J, Lewis J, Peter A, van Maris B, Ross A, Franssen E, et al. A
menopause-specific quality of life questionnaire: development and psychometric
properties. Maturitas 2008; 61 (1-2):107-121.
20.
Macías
J, Royuela A. La versión española del índice de la calidad de sueño de
Pittsburghh. Inf Psiquátricas 1996; 146: 465-472.
21.
Morin C, Belleville G, Belanger L, Ivers H.
The Insomnia Severity Index: psychometric indicators to detect insomnia cases
and evaluate treatment response. Sleep 2011; 34 (5): 601-608.
22.
Chiner E, Arriero J, Signes-Costa J, Marco J,
Fuentes I. Validation of the Spanish version of the Epworth Sleepiness Scale in
patients with a sleep apnea syndrome. Arch Bronconeumol 1999; 35 (9):422-427.
23.
Schmidt S, Vilagut G, Garin O, Cunillera O,
Tresserras T, Brugulat P, et al. Reference guidelines for the
12-Item Short-Form Health Survey version 2 based on the Catalan general
population. Med Clin (Barc.). 2012; 139(14): 613-625.
24.
Atkinson M, Sinha A, Hass S, Colman S, Kumar
R, Brod M, et al. Validation of a general measure of treatment satisfaction,
the Treatment Satisfaction Questionnaire for Medication (TSQM), using a
national panel study of chronic disease. Health Qual Life Outcomes 2004; 2: 12.
25.
Green S, Salkind N, editors. Using SPSS for
Windows and Macintosh, Books a la Carte. 8th Edition. TX (USA): Pearson; 2016.
26.
Aljumah K, Hassali M. Impact of pharmacist
intervention on adherence and measurable patient outcomes among depressed
patients: a randomised controlled study. BMC.Psychiatry 2015; 15: 219.
27.
Shaver J, Woods N. Sleep and menopause: a
narrative review. Menopause 2015; 22(8): 899-915.
28.
Jenabi E, Shobeiri F, Hazavehei S, Roshanaei
G. The effect of Valerian on the severity and frequency of hot flashes: A
triple-blind randomized clinical trial. Women Health 2018; 58(3): 297-304.
29.
Mirabi P, Mojab F. The effects of valerian
root on hot flashes in menopausal women. Iran J Pharm Res Winter 2013; 12(1):
217-222.
30.
Fahami F, Asali Z, Aslani A, Fathizadeh N. A
comparative study on the effects of Hypericum perforatum and passion
flower on the menopausal symptoms of women referring to Isfahan city health
care centers. Iran J Nurs Midwifery Res 2010; 15(4):202-207.
31.
Ghazanfarpour M, Sadeghi R, Abdolahian S,
Roudsari R. The efficacy of Iranian herbal medicines in alleviating hot
flashes: A systematic review. Int J Reprod Biomed (Yazd.) 2016; 14(3): 155-166.
32. Agan
K, Ozmerdivenli R, Degirmenci Y, Caglar M, Basbug A, Balbay E, et al. Evaluation
of sleep in women with menopause: results of the Pittsburg Sleep Quality Index
and polysomnography. J Turk Ger Gynecol Assoc 2015; 16(3): 149-152.
33. Pinkerton
J, Abraham L, Bushmakin A, Cappelleri J, Komm B. Relationship between changes
in vasomotor symptoms and changes in menopause-specific quality of life and
sleep parameters. Menopause 2016; 23(10): 1060-1066.
34.
Kalmbach D, Cheng P, Arnedt J,
Cuamatzi-Castelan A, Atkinson R, Fellman-Couture C, et al. Improving daytime
functioning, work performance, and quality of life in postmenopausal women with
insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction
therapy, and sleep hygiene education. J Clin Sleep Med 2019; 15 (7): 999-1010.
35.
Kok L, Kreijkamp-Kaspers S, Grobbee D, Lampe
J, van der Schouw T. Soy isoflavones, body composition, and physical
performance. Maturitas 2005; 52 (2):102-110.
36.
Riesco E, Choquette S, Audet M, Tessier D,
Dionne J. Effect of exercise combined with phytoestrogens on quality of life in
postmenopausal women. Climacteric 2011; 14(5): 573-580.
37. Fontvieille
A, Dionne I, Riesco E. Long-term exercise training and soy isoflavones to improve
quality of life and climacteric symptoms. Climacteric 2017; 20(3): 233-239.
38. Panvelkar
P, Saini B, Armour C. Measurement of patient satisfaction with community
pharmacy services: a review. Pharm World Sci 2009; 31(5): 525-537.

