ORIGINAL
Elevated D-Dimer and acute pulmonary embolism in COVID-19 patients
Dímero-D elevado y tromboembolismo pulmonar agudo en pacientes con COVID-19
Yoselin Dos Santos-Poleo1, Lorenzo Pérez-Sánchez1, Abrahams Ocanto2, Diana Oquillas-Izquierdo1, Francisco Rodríguez-Recio1
1Department of Diagnostic Imaging of Hospital General de Segovia, Segovia, Spain.
2Radiation Oncology Department of Hospital Universitario La Paz, Madrid, Spain.
* Autor para correspondencia.
|
|
Attribution-NonCommercial-ShareAlike 4.0 International License La revista no cobra tasas por el envío de trabajos, |
|
Abstract
Introduction. It has been determined that patients with SARS-CoV-2 infection and severe pneumonia with elevated D-dimer values can develop acute pulmonary thromboembolism (APE) as a complication, being one of the causes related to mortality in this group of patients.
Methods. A retrospective analysis of 12 patients diagnosed with SARS-CoV-2 infection with high clinical suspicion of APE confirmed by computed tomography pulmonary angiopgraphy (CTPA) was performed and the described findings are described.
Results. 12 patients with diagnosis of severe pneumonia, elevated D-dimer 9.2 μg / ml (1.4 - ˃20 μg / mL) and confirmation of SARS-CoV-2 infection through real-time reverse transcription polymerasa chain reaction (RT-PCR). APEs were observed mainly in segmental arteries (75%) and main arteries (25%). Pneumonia with patched areas of bilateral ground glass opacities was observed in 100% of the sample as a typical finding of SARS-CoV-2 infection.
Conclusion. SARS-CoV-2 infection is related to elevation of D-dimer and APE. The CTPA determines the diagnosis, severity and timely management (anticoagulation) of patients with APE. Therefore CTPA should be considered in all patients with elevated D-dimer or clinical worsening.
Keywords
COVID-19; embolism; CT scan; D-dimer; radiology
Resumen
Introducción. Se ha determinado que los pacientes con infección por SARS-CoV-2 y neumonía severa con valores elevados de dímero-D, pueden desarrollar tromboembolismo pulmonar agudo (TEP) como complicación, siendo una de las causas relacionada con la mortalidad en este grupo de pacientes.
Material y métodos. Se realizó un análisis retrospectivo de 12 pacientes con diagnóstico de infección por SARS-CoV-2 con alta sospecha clínica de APE confirmado por angio tomografia computarizada (AngioTC) y se describen los hallazgos descritos.
Resultados. 12 pacientes con diagnóstico de neumonía severa, dímero-D elevado 9,2 μg/ml (1,4 - ˃20 μg/ml) y confirmación de infección de SARS-CoV-2 a través de reacción en cadena de polimerasa reversa (RT-PCR). Se objetivaron TEP principalmente en arterias segmentarias (75%) y arterias principales (25%). En el 100% de la muestra se objetivó neumonía con áreas parcheadas de vidrio deslustrado bilaterales como hallazgo típico de infección por SARS-CoV-2.
Conclusión. La infección por SARS-CoV-2 está relacionada con elevación del dímero-D y con TEP. La angioTC determina el diagnóstico, severidad y manejo oportuno (anticoagulación) de los pacientes con TEP. Por tanto el angioTC debe ser considerado en todos los pacientes con dímero-D elevado o empeoramiento clínico.
Palabras clave
COVID-19; embolismo; CT scan; dímero-D; radiología
Introduction
Chinese scientists have isolated a new coronavirus: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019-nCoV) from hospitalized patients with severe pneumonia of unknown origin. Then in February 2020 it was designated as coronavirus disease 2019 (COVID-19) by WHO(1).
The clinical spectrum of SARS-CoV-2 infection is wide, including asymptomatic infection, mild respiratory disease course, and severe viral pneumonia that can be complicated by respiratory failure and even death(1,2).
Diagnosis is based on real-time reverse transcription polymerase chain reaction (RT-PCR) applied to the respiratory tract, with a sensitivity of 60-71%(3,4).
Imaging tests play an important role in the management of COVID-19 disease. According to a cohort of 1,014 patients in Wuhan, China, the sensitivity, specificity, and accuracy of chest-CT in detecting COVID-19 pneumonia was 97%, 25%, and 68%, respectively, using the RT-PCR results as reference(5).
The described patterns of COVID-19 viral pneumonia include: peripherally ground-glass opacities with multilobe and posterior involvement, bilateral distribution, and subsegmental vessel enlargement (˃3mm)(6).
Risk factors have been identified that help identify patients with poor prognosis at an early stage, such as older age, high SOFA score and D-dimer greater than 1μ / mL(1).
Elevated D-dimer is related to venous thromboembolism (VTE) and acute pulmonary embolism (APE), which is related to high mortality. However, the increase in D-dimer does not indicate the diagnosis of VTE / APE, it must be accompanied by high clinical suspicion and an imaging test that confirms such as computed tomography pulmonary angiography (CTPA)(7).
Zhou et al recently reported a positive correlation between elevated D-dimer levels and mortality in patients admitted with COVID-19(8).
Many studies have verified the elevation of D-dimer in patients with COVID-19, however they do not conclude if these patients have had APE. Both the Severe Acute Respiratory Syndrome (SARS) and the Middle East Respiratory Syndrome (MERS) are other similar epidemic-related coronaviruses, in which there is only one reported case of APE related to SARS, diagnosed by CTPA(9).
Therefore, the aim of this retrospective study was described the findings of APE diagnosed by CTPA in COVID-19 patients in Radiology Department of Hospital General de Segovia.
Methods
Study design and patients
This is a retrospective study conducted at the General Hospital of Segovia, Spain. During the study period among 320 patients with COVID-19 pneumonia were hospitalized. Twelve patients admitted for COVID-19 pneumonia were identified retrospectively, and they also presented an APE assessed by CTPA (Table 1). These patients were identified using the Radiology Information System (RIS) at the General Hospital of Segovia between March and April 2020. The diagnosis of COVID-19 pneumonia was made according to clinical guidelines published by the Ministerio de sanidad, consumo y bienestar social del gobierno de España(10). All the patients fulfilled the clinical criteria, elevated D-dimer and positive RT-PCR for COVID-19. 9/12 (75%) of the patients were admitted and anticoagulated at therapeutic doses with low molecular weight heparin (1mg / kg BID), 3/12 (25%) were not anticoagulated. Given the high clinical suspicion of APE, CTPA was performed on all patients.
Table 1. Patients characteristics
|
Characteristics |
Total (n=12) |
|
Age |
68,5 (57-90) |
|
Gender |
|
|
Male |
10 |
|
Female |
2 |
|
Time to CTPA (days) |
14 (10-21) |
|
D-dimer (μg/mL) |
9,2 (1,4 - ˃20) |
CTPA image acquisition
CTPA examination were performed in multi-detector CT scan (Philips Ingenuity Flex 32, Philips Medical Systems, Best, the Netherlands) by using a standard CTPA protocol. All patients were instructed to hold breath to minimize motion artifacts. The whole chest was scanned from lowest hemidiaphragm to the lung apex for each patient in the supine position. The tube voltage of 120 kV, tube current of 314 mA, collimation of 64x0.625mm, pitch of 0.937-1, table speed of 65mm / s, gantry rotation time of 0.75s. 80-100 mL nonionic iodinated contrast media (Iomeprol, Iomeron 300 Bracco, Milan, Italy) was injected into an antecubital vein at a flow rate of 4mL / s followed by a 25mL saline flush using a mechanical power injector. We used the automatic bolus tracking techique with a trigger threshold of 150 HU, and a fixed delay of 4s for optimal intraluminal contrast enhancement. The images were reconstructed with a thickness of 1-2mm. Finally the images were transmitted to post-processing workstations for multiplanar reconstruction and picture archiving and communication systems (PACS).
Image interpretation
All CTPAs were analyzed by Radiology Deparment attendings with 5 to 30 years of experience. The lung window was set as below: width, 1600 HU; level, -600HU. And CTPA images were analyzed on mediastinal window: width. 400 HU; level, 60 HU.
Statistical analysis
Stastical analysis was performed using IBM SPSS Statistics v. 23.0. All continuous variables were expressed as medians and ranges and categorical variables as counts and percentages.
Results
A total of 12 (100%) patients were diagnosed with SARS-CoV-2 infection by compatible clinic and RT-PCR (10 males / 2 females), with an average age of 68.5 years (range 57-90 years). All the patients presented severe clinical criteria of the disease and 9 (75%) were admitted to the hospital.
D-dimer was determined in the entire sample with an average of 9.2 µg / mL (range 1.4 - ˃20 µg / mL).
CTPA was performed in all patients with clinical suspicion of APE and elevated D-dimer approximately on day 14 (10 - 21) from symptom onset, with the finding of APE in 100%, distinguishing the following: 6 patients (50% ) had bilateral APE and 6 patients (50%) unilateral. In 9 patients (75%), the filling defects occurred in small branches for each lobe or segmental arteries, while in 3 patients (25%) they involved major arteries. The most frequently affected lobes were left upper lobe (50%) and left lower lobe (41.6%). Characteristics compatible with coronavirus pneumonia were also determined in 100% of patients: bilateral ground glass opacities with a predominance in the lower lobes and peripheral distribution with fibrous streaks (Figures 1-3).
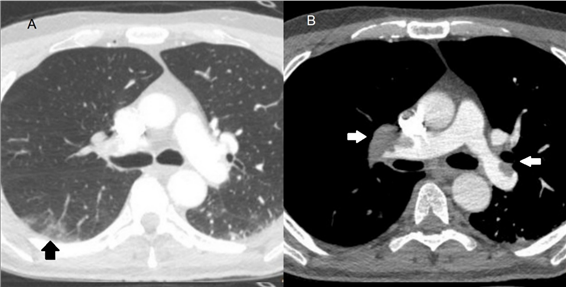
Figure 1. Images in a 61 year old man with COVID-19 pneumonia. A, axial chest CT in lung window obteined on day 10 after the onset of symtoms shows areas of peripheral ground-glass opacities (black arrow). B, axial CT pulmonary angiography demostrates bilateral filling defects (white arrows) involving main pulmonary arteries.
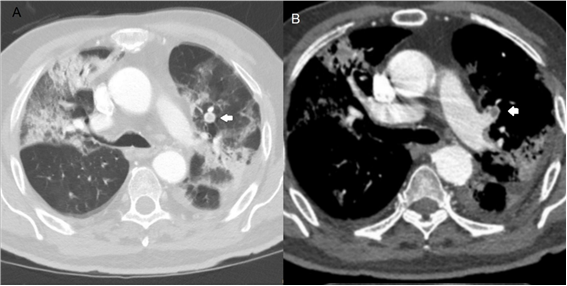
Figure 2. Images in a 90 year old man with COVID-19 pneumonia. A, axial chest CT in lung window obteined on day 12 after the onset of symptoms shows bilateral areas of peripheral ground-glass opacities, associated with crazy paving and consolidation; also its seen a filling defect in a lobar branch (white arrow). B, axial CT pulmonary angiography demostrates a filling defect involving lobar branch of the left upper lobe (white arrow).
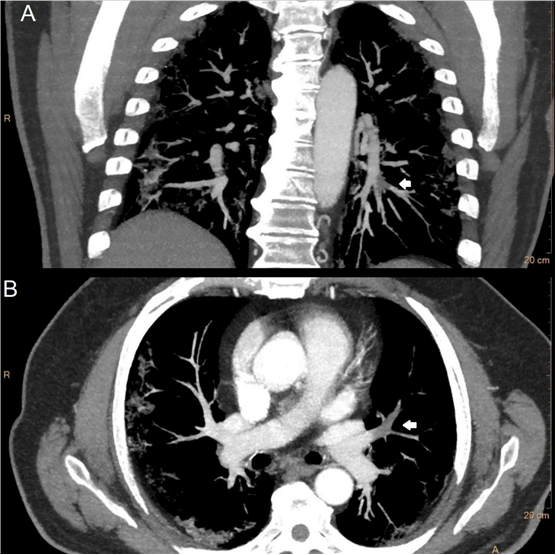
Figure 3. Images in a 65 year old man with COVID-19 pneumonia. A, coronal thick maximum intensity projection slab of CT pulmonary angiography demostrates a filling defect (white arrow) in a segmental arterie for the left lower lobe. B, axial thick maximum intensity projection slab of CT pulmonary angiography shows another filling defect (white arrow) involving lingular artery.
Nine patients (75%) were anticoagulated at therapeutic doses prior to performing the CTPA due to analitical criteria: elevation of D-dimer. While 3 patients (25%) were not anticoagulated. It should be noted that 2 patients in the sample presented symptoms of deep vein thrombosis (DVT), detected by ultrasonography before they underwent CTPA (Figure 4).
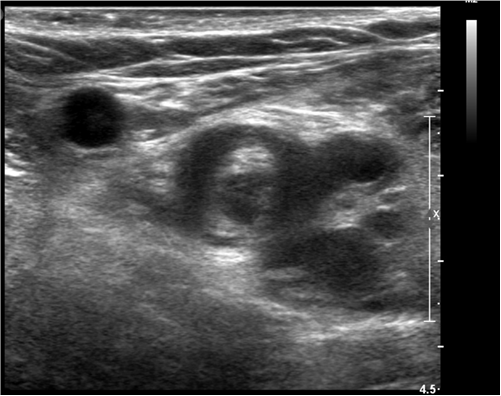
Figure 4. Ultrasound of the right lower limb in a 66 year old woman, who was diagnosed with COVID-19. The image shows soft intraluminal material and a non-compressible venous segment of the femoral vein as signs of acute deep vein thrombosis.
Discussion
Two coronaviruses previously known to cause epidemics have previously been the cause of severe lung damage and acute respiratory distress syndrome (MERS and SARS)(11). However, non case of APE in patients with MERS pneumonia has been documented and a single case of APE in a patient diagnosed with SARS was published(9).
Predisposition by sex to develop SARS-CoV-2 infection has been assessed and, according to what was published by Hua Cai(12), men have a greater predisposition due to a higher expression of ACE2 (SARS-CoV-2 receptor in pneumocytes), as shown in our study.
Multiple markers of poor prognosis have also been determined in SARS-CoV-2 infection, identifying: variations of troponin I, ferritin, lactate dehydrogenase (LDH) and interleukin-6 (IL-6) as the most important, adding the D-dimer and SOFA score elevated as the most important and those mainly associated with death(11).
It should be noted that the D-dimer rises in other circumstances such as DVT, cancer, peripheral vascular disease, pregnancy and inflammatory diseases(13).
In this article, 12 cases of patients with a diagnosis of SARS-CoV-2 and APE are documented, coinciding with compatible clinical conditions and elevation of D-dimer, 75% being anthocoagulated at therapeutic doses.
Imaging studies are vital to determine embolic complications related to D-dimer elevation. In this study, all cases of APE were observed through CTPA, therefore it is shown that patients with elevated D-dimer and COVID-19 pneumonia have a high occurrence rate of APE.
Unfortunately, only 2 patients (16.6%) underwent ultrasound of the lower limbs on suspicion of DVT, testing positive. Hence, prospective studies will be needed to determine the incidence of DVT in patients with SARS-CoV-2 infection.
There are other alternatives to conventional radiological imaging techniques such as nuclear medicine studies (ventilation / perfusion ganmagraphy) used in patients allergic to iodinated contrasts or even with high suspicion of APE not detected in CTPA(14).
According to what was published by Rotzinger et al, chest CT without contrast is a useful tool for diagnosing lung involvement in patients with suspected SARS-CoV-2 infection. However, patients with rapidly clinical worsening and / or elevated D-dimer at admission, may benefit from the use of contrast to determine APE(15). Another recent study by Grillet et al refers that the possibility of contrast enhanced CT rather than routine non contrast CT should be considered in COVID-19 patients with severe clinical features(16).
The characteristics of the lesions analyzed in CTPA reveal a greater predisposition of involvement of the lobar arteries and small segmental branches, which coincides with the work published by Jianpu Chen et al(7).
The mechanism by which patients with SARS-CoV-2 infection develop APE has not been determined. Data suggesting an origin of thrombhus in the lower limbs(9) that may be associated with reduced movement in patients predisposed to developing DVT have been found in autopsies of cadavers of patients infected with respiratory viruses.
Other studies have shown that thrombosis associated with influenza viral pneumonia is related to epithelial damage, endothelial cell and platelet dysfunction caused by infection(13). However, the pathophysiology of an elevated D-dimer with consequent APE in patients infected with SARS-CoV-2 is unknown today.
This study has multiple limitations: The study sample is low. Being a retrospective study limits the analysis of the data. The origin of the APE could not be determined (for example: lower limb). We suggest carrying out controlled studies to objectify the incidence of APE in patients with SARS-CoV-2 infection and thus introduce prophylactic measures that help to decrease the number of cases of APE and therefore decrease mortality, as well as therapeutic measures in the shortest possible time.
Conclusion
SARS-CoV-2
infection is related to elevations of D-dimer and APE. The CTPA determines the
diagnosis, severity and management (anticoagulation) of patients with APE.
Therefore, CTPA should be considered in all patients with elevated D-dimer or
clinical worsening, because APE is a life-threatening but potentially treatable
condition.
Acknowledgements
We thank all colleagues for helping us during the current study. We are also grateful to the many members of the the frontline medical staff for their selfness dedication in the face of this outbreak.
Compliance with ethical standards
Ethical approval (research with human and / or animal participants). The current study was conducted in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its subsequent amendments.
Referencias
1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3
2. Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin Exp Pediatr. 2020 Apr 2. doi: 10.3345/cep.2020.00493.
3. Yang Yang, Minghui Yang, Chenguang Shen, Fuxiang Wang, Jing Yuan, Jinxiu Li, Mingxia Zhang, Zhaoqin Wang, Li Xing, Jinli Wei, Ling Peng, Gary Wong, Haixia Zheng, Mingfeng Liao, Kai Feng, Jianming Li, Qianting Yang, Juanjuan Zhao, Zheng Zhang, Lei Liu, Yingxia Liu. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. doi: https://doi.org/10.1101/2020.02.11.20021493.
4. Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology. 2020 Feb 27:200527. doi: 10.1148/radiol.2020200527.
5. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 Feb 26:200642. doi: 10.1148/radiol.2020200642.
6. Damiano Caruso, Marta Zerunian, Michela Polici, Francesco Pucciarelli, Tiziano Polidori, Carlotta Rucci, Gisella Guido, Benedetta Bracci, Chiara de Dominicis, Prof. Andrea Laghi. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020. https://doi.org/10.1148/radiol.2020201237.
7. Chen, Jianpu and Wang, Xiang and Zhang, Shutong and Liu, Bin and Wu, Xiaoqing and Wang, Yanfang and Wang, Xiaoqi and Yang, Ming and Sun, Jianqing and Xie, Yuanliang, Findings of Acute Pulmonary Embolism in COVID-19 Patients (3/1/2020). Available at SSRN: https://ssrn.com/abstract=3548771 or http://dx.doi.org/10.2139/ssrn.3548771.
8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3.
9. Ng, K. H., Wu, A. K., Cheng, V. C., Tang, B. S., Chan, C. Y., Yung, C. Y., Luk, S. H., Lee, T. W., Chow, L., & Yuen, K. Y. (2005). Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgraduate medical journal J. 2005; 81: 1-3. https://doi.org/10.1136/pgmj.2004.030049.
10. Ministerio de Sanidad, Consumo y Bienestar Social del Gobierno de España. Dirección general de salud pública, calidad e innovación. Centro de coordinación de alertas y emergencias sanitarias. Procedimiento de actuación frente a casos de infección por el nuevo coronavirus (SARS-CoV-2). 11 de abril de 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Procedimiento_COVID_19.pdf (accessed April 12, 2020).
11. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5.
12. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020 Apr;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X.
13. Visseren FLJ, Bouwman JJM, Bouter KP, Diepersloot RJA, De Groot G, Erkelens DW. Procoagulant Activity of Endothelial Cells after Infection with Respiratory Viruses. Thromb Haemost 2000; 84(02): 319-324. DOI: 10.1055/s-0037-1614014.
14. Zuckier LS, Moadel RM, Haramati LB, Freeman L. Diagnostic evaluation of pulmonary embolism during the COVID-19 pandemic. J Nucl Med. 2020 Apr 1. pii: jnumed.120.245571. doi: 10.2967/jnumed.120.245571.
15. D.C. Rotzinger, C. Beigelman-Aubry,C. von Garnier, and S.D. Qanadli. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb Res. 2020 Jun; 190: 58–59. doi: 10.1016/j.thromres.2020.04.011.
16. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected by Pulmonary CT Angiography. Radiology. 2020 Apr 23:201544. doi: 10.1148/radiol.2020201544.
