ORIGINAL
The effect of Valsalva and Jendrassik maneuvers on acoustic reflex
El efecto de las maniobras de Valsalva y Jendrassik sobre el reflejo acústico
Deniel Fakouri1, Mohammad Hosein Taziki Balajelini2, Seyed Mehran Hosseini3
1 Golestan University of Medical sciences, Student Research Committee, International Campus, School of Medicine, Golestan University of Medical Sciences, Gorgan 4934174515, Golestan, Iran
2 MD., Golestan University of Medical Sciences, Department of Otolaryngology, School of Medicine, Golestan University of Medical Sciences, Gorgan 4934174515, Golestan, Iran
3 MD. PhD, Golestan University of Medical Sciences, Department of Physiology, School of Medicine, Golestan University of Medical Sciences, Gorgan 4934174515, Golestan, Iran. Neuroscience Research Center, School of Medicine, Golestan University of Medical Sciences, Gorgan 4934174515, Golestan, Iran
* Corresponding Author.
|
|
Attribution-NonCommercial-ShareAlike 4.0 International License La revista no cobra tasas por el envío de trabajos, |
|
Abstract
Aims. Studying modulation of acoustic reflex by the Valsalva and the Jendrassik manoeuvres
Settings and Design. Quasi-experimental research with the pre-post testing design
Methods and Material. This self-control study was conducted on 12 healthy college students aged 21.23 ± 2.02 years. They were well trained to perform the standard Valsalva manoeuvre: maintenance of a 15-second expiratory pressure at 40 mmHg with open glottis. The Jendrassik manoeuver was performed by pulling clenched hands. Acoustic reflex threshold, acoustic reflex decay, middle ear pressure and compliance were measured before and after the Valsalva and the Jendrassik manoeuvres. The order of the right or the left ear recording was random. The recovery time following manoeuvres were 5 minutes.
Statistical analysis used All variables were compared using the repeated measured ANOVA.
Results. There were no statistical differences in the threshold and in the decay of acoustic reflex before and after the Valsalva and the Jendrassik manoeuvres. This finding was similar in both ears and had no laterality.
Conclusions. The Valsalva and the Jendrassik manoeuvres as modulator of some somatic and autonomic reflexes including the sound induced startle reflex have no effect on acoustic reflex.
Keywords
Valsalva manoeuvre (VM); Acoustic reflex threshold (ART); Acoustic reflex decay (ARD); Startle reflex
Resumen
Objetivos. Estudiar la modulación del reflejo acústico mediante las maniobras de Valsalva y Jendrassik.
Configuración y Diseño: Investigación cuasi experimental con el diseño de prueba previa y posterior
Materiales y Métodos. Este estudio de autocontrol se realizó en 12 estudiantes universitarios sanos de 21.23 ± 2.02 años. Estaban bien entrenados para realizar la maniobra estándar de Valsalva: mantenimiento de una presión espiratoria de 15 segundos a 40 mmHg con una glotis abierta. La maniobra de Jendrassik se realizó tirando de las manos apretadas. El umbral del reflejo acústico, la disminución del reflejo acústico, la presión del oído medio y el cumplimiento se midieron antes y después de las maniobras de Valsalva y Jendrassik. El orden de la grabación del oído derecho o izquierdo fue aleatorio. El tiempo de recuperación después de las maniobras fue de 5 minutos.
Análisis Estadístico utilizado. Todas las variables se compararon utilizando el ANOVA medido repetidamente
Resultados. No hubo diferencias estadísticas en el umbral y en la decadencia del reflejo acústico antes y después de las maniobras de Valsalva y Jendrassik. Este hallazgo fue similar en ambos oídos y no tenía lateralidad.
Conclusiones. Las maniobras de Valsalva y Jendrassik como moduladores de algunos reflejos somáticos y autónomos, incluido el reflejo de sobresalto inducido por el sonido, no tienen efecto sobre el reflejo acústico.
Palabras clave
Maniobra de Valsalva (VM); umbral del reflejo acústico (ART); decaimiento del reflejo acústico (ARD); reflejo de sobresalto
Contribution to scientific literature
Auditory stimulus can induce involuntary reflex in the middle ear. This reflex is named the acoustic reflex. An overlap exists among some neural centers that involve in processing of auditory signals and the neural centers that activates during the voluntary contractions of Valsalva and Jendrassik manoeuvres.
Valsalva manoeuvre change the activity of the cerebellum, brain stem, limbic system, amygdala and some cortical regions with different time courses, and unexpectedly has laterality and affects central nervous system in one side much more than the opposite side. Another instance is the Jendrassik manoeuvre that reinforces the stretch reflex and has peripheral and central effects on sympathetic tone. In our knowledge there is no report about the effect of these manoeuvres on stapedius muscle reflex as the smallest striated skeletal muscle of the body.
Introduction
Middle ear functions include converting sound energy into kinetic mechanical energy, amplifying sound pressure, resonance and increasing sensitivity to certain frequencies. Middle ear also protects nervous system against harsh sounds. The mechanism of the middle ear protection is by means of decreasing the energy transmission of intense sounds to the inner ear. This phenomenon is involuntary and is done by the acoustic reflex (AR). The auditory stimulus depending on its intensity can induced two responses, the startle reflex and the acoustic reflex. In humans the AR is mainly caused by stapedius muscle contraction(1). The tensor tympani muscle has no important role in human AR. The contraction of tensor tympani is due to non-acoustic stimuli such as facial stimulation, tight eyes closing, swallowing and also to auditory stimulus e.g., the startles response(2). The AR neural pathways include the vestibulocochlear nerve, the cochlear nucleus, the superior olivary complex, and the facial nerve. The central pathways of auditory signals can affect the autonomic activity of the amygdala, hippocampus, and anterior cingulate cortex(3-5). The stapedius muscle is innervated by the facial nerve. Functionally, the facial nerve contains the following components: somatic afferent (general sensory), general visceral efferents (parasympathetic), general visceral afferents, special visceral afferent (special visceral senses taste and smell), special somatic afferent (special senses related to body position) and special visceral efferents (branchiomotor). These special visceral efferents supply the motor nerve of the stapedius muscle(6-8).
All activities that have some strain are very similar to Valsalva manoeuvre (VM). Cardiovascular effects of VM and its effect on autonomic balance are well documented(9,10). The VM is an effective ways for increasing the parasympathetic activity and has clinical application in the control of supraventricular tachycardia. There is very limited evidence regarding the effect of the autonomic system on skeletal muscle reflex. However, the proposed assumptions for such effects include the autonomic modulations on muscle spindle and on the muscle blood flow(11-13).
Jendrassik manoeuvre (JM) also has clinical application. JM intensifies some muscle reflex by activation of muscle spindle through pre- and/or post-synaptic mechanisms(14). All forms of exertion, including anaerobic exercise, defecation, and lifting or moving heavy objects induce physiological effects that are very similar to VM. In patients with middle ear problems such as inflammatory otitis and Eustachian tube disorder, the autoinflation maneuver may inadvertently be associated with varying degrees of strain and VM-like conditions(15). However, there is very limited data regarding the effect of VM and JM on muscle reflex and in our knowledge there is no report about the effect of JM and VM on stapedius muscle. The stapedius muscle is the smallest skeletal muscle in the body and its length is about one millimeter. This skeletal muscle in humans lacks voluntary control and, unlike other skeletal muscles, its reflex contraction is not dependent on direct or painful mechanical stimulation. This muscle has a special visceral efferent innervation and its contraction protects the inner ear against loud sounds. The AR as a popular audiology test can also provide quantitative information about the stapedius contraction. In this study the modulation of acoustic reflex by VM and JM were investigated using the AR test.
Subjects and Methods
This quasi-experimental and pre-post study was self-controlled. The participants were 12 male volunteer students. The study protocol was confirmed by the research council of Golestan University of Medical Sciences. The ethic number was ir.goums.REC.1396.275. All participants were informed about the study and assigned the informed consent. All participants had good general health and had no history of disease, any drug use, any ear disease or hearing impairment. The participants underwent the general clinical and otoscopic examination. There was not any exclusion of the case from this study because of a closed ear canal, rigid ear wax, rupture of the tympanum, a history of Dizziness, vertigo and tinnitus. All experiments were performed in the morning and between 9 and 11AM. The volunteers were fasting at least 3 hours before the experiment. The subjects were test in sitting position. They were trained to remain relaxed, not to move the head, nor to swallow during the audiometric tests. The pure tone audiometry, AR and tympanometry was performed by Impedance Audiometer AT235 version 1.1, Model 1077, Interacoustics A/S; Denmark. It was calibrated for Gorgan city based on its high from the sea level. The AR was first established, using ipsilateral tonal stimulation at frequencies of 500, 1,000 and 2,000 Hz. We used auto mode for acoustic reflex test. This mode had five intensity steps. If the AR at selected frequency was elicited twice at the set-threshold then the remaining steps would be stopped automatically. The stimulus intensity for each frequency was 80, 85, 90, 95 and 100 dB(16). The frequency of sound stimulus in AR decay test was 1000 Hz. For every participant, the tympanometry and the AR tests were repeated immediately following the performance of VM and/or JM. Each participant performed the VM for 15 seconds by expiring through a plastic tube that was connected to a mercury column. The target expiratory pressure with open glottis was set at 40 mmHg. The tube had a small hole to prevent pressure from easily increasing at the mouth without any intrathoracic hemodynamic effects(17). The JM was done by clenched hand pulling which consists of pulling the hands apart against interlocked fingers(18). Mean AR threshold, AR decay, middle ear pressure and compliance were measured before and after the VM and JM. The pure tone audiometry was done for all participants. The test frequency range was 250-8K Hz.
All data were reported as mean (SD). The normal distribution of data was checked by the Shapiro-Wilk test. All variables were compared using the repeated measured ANOVA. The Mann-Whitney U test was used for non-parametric data (e.g., the left and right sides). The statistical analysis was computed by SPSS 16 statistical software.
Results
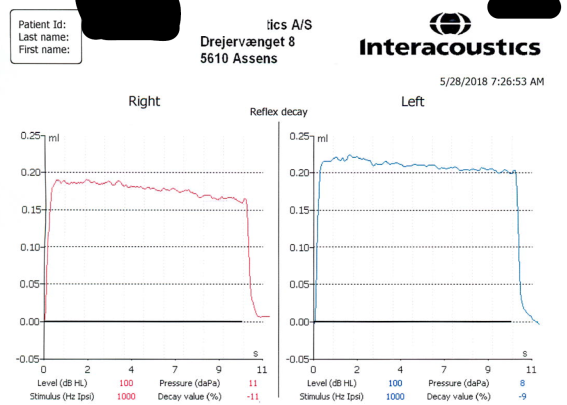
The mean (SD) of age, weight and height of participants were 21.23 (2.02) years, 71.21 (6.45) kilograms and 174.57 (5.3) centimeter respectively. A sample of tympanometry and AR tests were showed in Figure 1. A sample of AR decay test was showed in Figure 2.
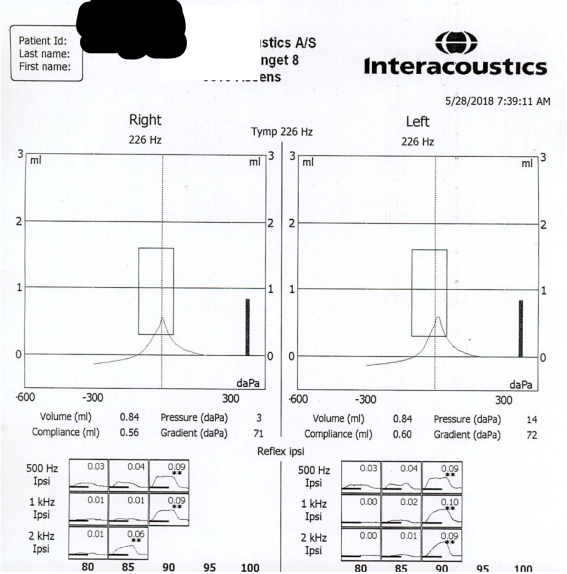
Figure 1. A sample of tympanometry and ipsilateral acoustic reflex tests. The first row represents the tympanometry of right and left ear. The second raw represents the acoustic stimulus frequency in vertical axis and its intensity in the horizontal axis. The induced and the replicable acoustic reflex are indicated by two star marks. The numbers show the deflection values of middle ear compliance in ml units.
![]()
Figure 2. A sample of acoustic reflex decay test at basal condition. It represented the percent of decay of striatal muscle contraction. This contraction was induced by the 1000 Hz acoustic stimulus. The duration and the intensity of acoustic stimulus were10 seconds and 10 dB over the acoustic reflex threshold respectively.
The mean (SD) of middle ear compliance, middle ear volume, pressure gradient and AR decay at basal resting condition and after VM and JM are shown in Table 1. There were no significant changes in these variables before and after the VM or JM. All pure tone audiometry were normal and there was not any hearing loss.
Table 1. The mean (SD) of acoustic reflex (AR) decay and middle ear compliance, pressure, volume and gradient at basal resting condition and after Valsalva and Jendrassik manoeuvres, n=12.
|
variable |
Right |
Left |
||||
|
Basal |
Valsalva |
Jendrassik |
Basal |
Valsalva |
Jendrassik |
|
|
AR decay (%) |
-12.5 (5.72) |
-26.83 (28.9) |
-20.2 (16.7) |
-14.5 (6.95) |
-22.25 (29.23) |
-21.2 (17.6) |
|
Compliance (ml) |
0.57 (0.15) |
0.54 (0.11) |
0.58 (0.14) |
0.65 (0.25) |
0.65 (0.27) |
0.69 (0.28) |
|
Volume (ml) |
1.22 (0.29) |
1.27 (0.31) |
1.39 (0.35) |
1.11 (0.26) |
1.15 (0.32) |
1.18 (0.25) |
|
Gradient (daPa) |
79.17 (6.31) |
80.17 (13.29) |
88.80 (14.65) |
79.67 (29.05) |
75 (22.47) |
82.80 (29.16) |
Discussion
These data indicated that 1000 Hz sound stimulation with 10±5 dB above the acoustic reflex threshold induced less than 15 percent decay in AR in normal healthy ears during 10 seconds. This fatigue was similar in left and right sides. Following the VM and JM this AR decay was changed to more negative values. However, these decreases were not statistically significant. Our data in resting or basal condition were similar to other reports(19). As expected, the Valsalva and the Jendrassik manoeuvres had no effect on the compliance, pressure, gradient and volume of the middle ear. It should be noted that the VM is not equivalent to autoinflation. Autoinflation means an increase in the pressure of the middle ear by the mechanism of air transfer through the Eustachian tube which is done by the person. Although not widely accepted, autoinflation is clinically practiced in some cases of serous otitis(20). The VM is a forced expiration against the closed glottis and is not associated with pressure transfer to the middle ear; VM induces hemodynamic changes and is associated direct and indirect autonomic reflexes during a 4-phase step(21). There are some evidences regarding the autonomic modulation of skeletal muscle contraction(22,23). Central or peripheral effects on muscle spindle discharges as well as the possibility of altering muscle blood flow are mechanisms that proposed for autonomic effects on skeletal muscle stretch reflex(24,25).
The autonomic effects of VM are well known. Cooke reported a baroreflex independent effect of VM on autonomic tone(26). However, the effects of VM on skeletal muscle reflex and its mechanisms are still not completely clear. León-Sarmiento reported the effect of VM on corneal or blink reflex. They proposed an inhibitory effect of VM on muscle spindle efferents(27). Loud sound can induce blink reflex by activation of facial nerve. This polysynaptic reflex has similarities to the AR(28). We did not observe any significant changes in AR decay following VM and JM. This may be related to the time course and size effect of VM or JM on AR. The time of AR decay test is 10 seconds and the autonomic effect of VM in phase 1 and 3 is very short(10,21). Therefore, a transient and short-lasting effect of VM or JM may be missed in AR decay test. The JM can increase the sensitivity of muscle spindle and can decrease the tonic pre-synaptic inhibition of the alpha motor neuron. The effect of JM on stretch reflex is very fast and transient (29). The main effect of JM is to increase the muscle spindle's sensitivity; therefore if the stapedius lacks this sensory receptor, JM will not alter its reflex contraction. We did not find any information regarding the stapedius muscle spindle in literature. The JM also has complex transcortical inhibitory effects on muscle stretch reflex. Our data may also be interpreted as a manifestation of these late inhibitory effect of JM on muscle reflex. However, this effect is reported in soleus and tibialis anterior muscles(30).
This study had some limitations and any generalization of data need more caution. They included small sample size, using only auto mode method for AR study and no simultaneous EMG recording due to technical and laboratory limitations. The application of a wideband reflectance technique instead of single probe tone for AR and a larger sample size can provide better data about the effect of VM and JM on AR.
Acknowledgement
The authors wish to thank all volunteer students who participated in the study.
Conflict of interest
The authors declare no conflict of interest financial or otherwise.
Referencias
1. Schairer KS, Feeney MP, Sanford CA. Acoustic reflex measurement. Ear and hearing. 2013;34:43s-7s
2. Rainsbury JW, Aron M, Floyd D, Bance M. Vocalization-Induced Stapedius Contraction. Otology & Neurotology. 2015;36(2):382-5.
3. Deuter CE, Kuehl LK, Blumenthal TD, Schulz A, Oitzl MS, Schachinger H. Effects of cold pressor stress on the human startle response. PloS one. 2012;7(11):e49866.
4. Pissiota A, Frans Ö, Michelgård Å, Appel L, Långström B, Flaten MA, et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. European Journal of Neuroscience. 2003;18(5):1325-31.
5. Weber M, Schnitzler HU, Schmid S. Synaptic plasticity in the acoustic startle pathway: the neuronal basis for short-term habituation? European Journal of Neuroscience. 2002;16(7):1325-32.
6. Susan Standring. Development of the nervous system. In: Standring S, Anand N, Birch R, Collins P, Crossman AR, Gleeson M, Jawaheer G, Smith A, Spratt JD, Stringer MD, Tubbs RS, Tunstall R, Wein AJ, Wigley CB., editor. Gray's Anatomy E-Book: The Anatomical Basis of Clinical Practice. 41st ed: Elsevier: 2016: 238-270
7. Duggan WF. Anatomy of the Eye and Orbit. In: Forrester JV, Dick, A. D., McMenamin, P. G., Roberts, F., Pearlman, E., editor. The Eye E-Book: Basic Sciences in Practice. 4th ed: Edinburgh: Saunders; 2016:723-4.
8. Francis HW. Anatomy of the temporal bone, external ear, and middle ear. In: Flint PW HB, Lund V, Niparko JK, Robbins KT, Thomas JR, Lesperance MM., editor. Cummings Otolaryngology–Head and Neck Surgery. 6th ed: Saunders; 2015:1977-86.
9. Smith G, Broek A, Taylor DM, Morgans A, Cameron P. Identification of the optimum vagal manoeuvre technique for maximising vagal tone. Emerg Med J. 2015;32(1):51-4
10. Hosseini S.M., Jamshir M. Valsalva Maneuver and Strain-Related ECG Changes. Res Cardiovasc Med. 2015; 4(4): e28136.
11. Roatta S, Windhorst U, Ljubisavljevic M, Johansson H, Passatore M. Sympathetic modulation of muscle spindle afferent sensitivity to stretch in rabbit jaw closing muscles. The Journal of Physiology. 2002;540(1):237-48.
12. Radovanovic D, Peikert K, Lindström M, Domellöf FP. Sympathetic innervation of human muscle spindles. Journal of Anatomy. 2015;226(6):542–8.
13. Heaton JT, Sheu SH, Hohman MH, Knox CJ, Weinberg JS, Kleiss IJ, et al. Rat whisker movement after facial nerve lesion: evidence for autonomic contraction of skeletal muscle. Neuroscience. 2014;265:9-20.
14. Ertuglu LA, Aydin A, Kumru H, Valls-Sole J, Opisso E, Cecen S, Türker KS. Jendrassik maneuver effect on spinal and brainstem reflexes. Exp Brain Res. 2019 ;237(12):3265-3271. doi: 10.1007/s00221-019-05668-y. PMID: 31650212.
15. Perera R, Glasziou PP, Heneghan CJ, McLellan J, Williamson I. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2013; 31;(5):CD006285. doi: 10.1002/14651858.CD006285.pub2. PMID: 23728660.
16. Keefe DH, Feeney MP, Hunter LL, Fitzpatrick DF. Aural Acoustic Stapedius-Muscle Reflex Threshold Procedures to Test Human Infants and Adults. J Assoc Res Otolaryngol. 2017;18(1):65-88. doi: 10.1007/s10162-016-0599-z. PMID: 27957612; PMCID: PMC5243268.
17. Junqueira LF Jr. Teaching cardiac autonomic function dynamics employing the Valsalva (Valsalva-Weber) maneuver. Adv Physiol Educ. 2008 Mar;32(1):100-6. doi: 10.1152/advan.00057.2007. PMID: 18334576.
18. Nardone A, Schieppati M. Inhibitory effect of the Jendrassik maneuver on the stretch reflex. Neuroscience. 2008;15;156(3):607-17. doi: 10.1016/j.neuroscience.2008.07.039. PMID: 18713647.
19. Rosenhall U, Lidén G, Nilsson E. Stapedius reflex decay in normal hearing subjects. J Am Audiol Soc. 1979;4(4):157-62. PMID: 422428.)
20. Han JJ, Park JM, Kim DK, Park SY, Park SN. A pilot study to investigate the therapeutic effect of Valsalva maneuver on otitis media with effusion in adults. Auris Nasus Larynx. 2019;46(1):34-37. doi: 10.1016/j.anl.2018.05.012. PMID: 29914826.
21. Pstras L, Thomaseth K, Waniewski J, Balzani I, Bellavere F. The Valsalva manoeuvre: physiology and clinical examples. Acta Physiol (Oxf). 2016;217(2):103-19. doi: 10.1111/apha.12639.. PMID: 26662857.
22. Roatta S, Windhorst U, Ljubisavljevic M, Johansson H, Passatore M. Sympathetic modulation of muscle spindle afferent sensitivity to stretch in rabbit jaw closing muscles. J Physiol. 2002;540(Pt 1):237-48. doi: 10.1113/jphysiol.2001.014316. PMID: 11927683; PMCID: PMC2290222.
23. Rodrigues ACZ, Messi ML, Wang ZM, Abba MC, Pereyra A, Birbrair A, Zhang T, O'Meara M, Kwan P, Lopez EIS, Willis MS, Mintz A, Files DC, Furdui C, Oppenheim RW, Delbono O. The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta Physiol (Oxf). 2019 ;225(3):e13195. doi: 10.1111/apha.13195. Epub 2018 Oct 22. PMID: 30269419; PMCID: PMC7224611.
24. Radovanovic D, Peikert K, Lindström M, Domellöf FP. Sympathetic innervation of human muscle spindles. J Anat. 2015;226(6):542-8. doi: 10.1111/joa.12309. PMID: 25994126; PMCID: PMC4450958.
25. Birznieks I, Boonstra TW, Macefield VG. Modulation of human muscle spindle discharge by arterial pulsations--functional effects and consequences. PLoS One. 2012;7(4):e35091. doi: 10.1371/journal.pone.0035091. Epub 2012 Apr 17. PMID: 22529975; PMCID: PMC3328488.
26. Cooke WH, Carter JR, Kuusela TA. Muscle sympathetic nerve activation during the Valsalva maneuver: interpretive and analytical caveats. Aviat Space Environ Med. 2003;74(7):731-7. PMID: 12862327
27. León-Sarmiento FE, Martín-Torres MD. El reflejo orbicular de los ojos (R3) y la maniobra de Valsalva [Orbicular eye reflex (R3) and the valsalva manoeuvre]. Rev Neurol. 2001 Jun 1-15;32(11):1020-2. Spanish. PMID: 11562821.
28. Jerath N, Kimura J. F wave, A wave, H reflex, and blink reflex. Handb Clin Neurol. 2019;160:225-239. doi: 10.1016/B978-0-444-64032-1.00015-1.
29. Ertuglu LA, Karacan I, Yilmaz G, Türker KS. Standardization of the Jendrassik maneuver in Achilles tendon tap reflex. Clin Neurophysiol Pract. 2017;3:1-5. doi: 10.1016/j.cnp.2017.10.003. PMID: 30214998; PMCID: PMC6133913.
30. Nardone A, Schieppati M. Inhibitory effect of the Jendrassik manoeuvre on the stretch reflex. Neuroscience 2008;156(3):607-617
