EDITORIAL (English version)
Pharmacogenetics and mental disorders
Farmacogenética y trastornos mentales
Ignacio Jáuregui-Lobera
Instituto de Ciencias de la Conducta y Universidad Pablo de Olavide. Sevilla. España
* Autor para correspondencia.
|
|
Attribution-NonCommercial-ShareAlike 4.0 International License La revista no cobra tasas por el envío de trabajos, |
|
Pharmacogenetics involves the study of single gene mutations and their effect on drug response, analyzing DNA structural variations and impact on drug metabolism, efficacy and tolerability based on the fact that DNA remains stable and does not change with time or age. Polymorphic variation in the genes that encode the functions of transporters, metabolizing enzymes, receptors, and other proteins can result in individual differences in the dose-plasma concentration response relationships for many important therapeutic agents. A much broader term refers to pharmacogenomics, which focuses on surveying the entire genome to assess several determinants of drug responses. Its study includes genome-wide variations and potential complex inter-actions as well as alterations in gene expression and post-translational modifications (e.g. proteomics) that correlate with drug response(1,2). Overall, pharmacogenetics deals with single genes and their structure while pharmacogenomics relates to gene function influenced by the environment, both can play a role in human disease including drug metabolism(3).
The advent of pharmacogenomics began this century following the completion of the human genome in 2003, and the ready availability of new genotyping and sequencing technologies, which have enabled the assessment of the whole genome(3).
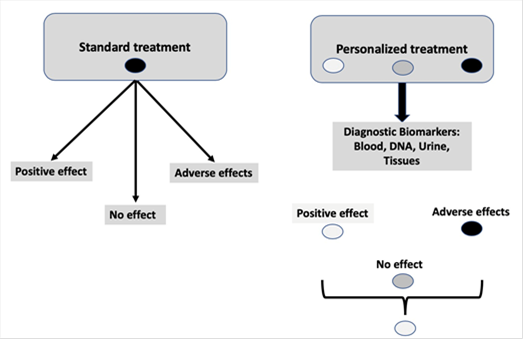
Figure 1. Standard treatment vs personalized treatment
Pharmacogenetics uses the patient's genetic information to select the most appropriate pharmacological treatment, maximizing therapeutic efficacy and minimizing adverse effects. In this regard pharmacogenetics aims to practice a personalized medicine. Pharmacogenetics makes it easier for the doctor to select between different pharmacological alternatives in a personalized way, it allows the recommendation of specific doses for each patient, and it helps to anticipate information on pharmacological safety. In addition, it permits to increase de number of patients who respond to a specific treatment and it helps to reduce the time necessary to select the most appropriate treatment in each case, thus improving the patients’ quality of life(4,5). Figure 1 shows the basic idea of this approach.
Pharmacogenetics is most often based on the cytochrome P450 enzyme system, primarily found in the liver and involves genes coding for the production of cytochrome P450 enzymes. The response to medications depends on each individual’s ability to metabolize drugs with most drugs broken down by this enzyme system dependent on the genetic makeup of each person. Approximately 90% of all drugs are metabolized by seven cytochrome enzymes (CYP1A2, CYP3A4, CYP3A5, CYPC19, CYP2D6, CYP2C9 and CYP2B6). In the case of CYP2D6 (the CYP2D7 gene is located on chromosome 22ql3.1), it contributes to the breakdown or oxidative metabolism of 25% of most commonly prescribed medications. Figure 2.
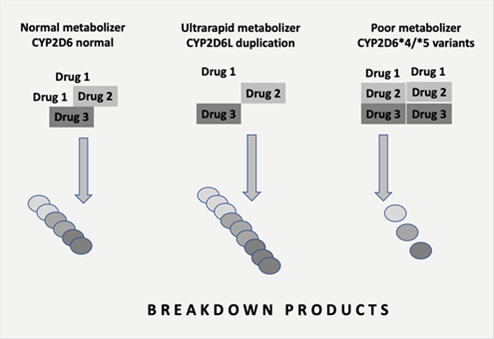
Figure 2. Normal, ultrarapid and poor metabolizers
The CYP2D7 gene has over 130 genetic variants with disturbances including duplications, single nucleotide polymorphisms, splice defects, deletions and frame shift mutations. As result of this complexity, a decreased, increased or nonfunctional enzyme activity may be found. In addition, cytochrome P450 enzymes may be altered by the environment in the form of inhibitors or inducers as well as impacted by drug-drug interactions(3).
Pharmacogenetics testing in order to optimize treatments is relevant in mental health where 20% of the 121 pharmacogenetics markers are recognized by the FDA as informative for clinical practice involving psychiatric drugs(6).
Thus, a personalized prescription of drugs in mental health would result in:
· A reduction of financial and personal costs linked to adverse drug effects.
· Less time to select the most appropriate medication and doses.
· Less adverse drug reactions.
· Less emergency visits.
· Less medications prescribed.
By means of this approach, it is possible to identify whether an individual has a normal, rapid, or poor metabolizer status before a specific drug is prescribed. In this regard, practitioners would be more selective with a greater assurance of success based on an individual’s genetic pattern(6).
In Spain the AB-GEN study, including 316 patients with major depression, was designed to evaluate the efficacy of Neurofarmagen® (a medical platform specifically designed to provide a personalized medicine in the field of psychiatry based on the pharmacogenetic analyses) in order to select the most appropriate treatment. The efficacy was 51.3% (Neurofarmagen®) vs 36.1% (standard treatment). With respect to adverse effects after a treatment of 12 weeks, the percentages of patients free of those effects were 68.5% (Neurofarmagen®) vs 51.4% (standard treatment). Neurofarmagen® includes more than 20 antidepressants, 12 antipsychotic drugs, several anxiolytics, mood stabilizers, anticonvulsants and others such as Methylphenidate or Atomoxetine(5).
In other study (GENEPSI study), Espadaler et al. found that the use of Neurofarmagen® reduced the number of non-stabilizer patients in 40% regardless the mental disorder. This study included 182 patients with different mental disorders with a follow-up of 12 weeks(7).
Nowadays the growing list of personalized testing measures besides cytochrome P450 enzyme genes often includes other genes that play a role in the field of mental disorders (neurotransmitter receptors, transporters, metabolic enzymes, ion channel functions). All these genes play a role in pharmacodynamic aspects of psychiatric medications. With this new approach psychiatrists and psychologists can use their knowledge-base and experience with mental disorders in the context of pharmacogenetics to help patients and clinicians make better decisions and choices regarding treatment plans and selection of medication(3).
Referencias
1. McCarthy AD, Kennedy JL, Middleton LT. Pharmacogenetics in drug development. Philos Trans R Soc Lond B Biol Sci. 2005;360:1579-1588.
2. Lesko LJ, Salerno RA, Spear BB, Anderson DC, Anderson T, Brazell C, et al. Pharmacogenetics and pharmacogenomics in drug development and regulatory decision making: Report of the first FDA-PWG-PhRMA-Dru Safe Workshop. J Clin Pharmacol. 2003;43:342-358.
3. Butler MG. Pharmacogenetics and psychiatric care: A review and commentary. J Ment Health Clin Psychol. 2018; 2:17-24.
4. Pirmohamed M. Pharmacogenetics: past, present and future. Drug Discov Today. 2011;16: 852-861.
5. Pérez V, Salavert A, Espadaler J, Tuson M, Saiz-Ruiz J, Sáez-Navarro C, et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depression disorder: results of a randomized, double-blind clinical trial. BMC Psychiatry. 2017;17:250.
6. Hamilton SP. The promise of psychiatric pharmacogenomics. Biol Psychiatry. 2015; 77:29-35.
7. Spadaler J, Tuson M, López-Ibor JM, López-Ibor F, López-Ibor MI. Pharmacogenetic testing of the guidance of Psychiatric treatment: A multicenter retrospective analysis. CNS Spectr. 2016;21:1-10.