ORIGINAL
Influence of olive polyphenols on glucose and cholesterol levels in medaka fish
Influencia de los polifenoles del olivo en los niveles
de glucosa y colesterol en el pez medaka
Luís Torró Montell1, Ernesto Cortés-Castell2, Carmen Veciana-Galindo1, Eliana Sirvent-Segura1, Francisco Gil-Guillén3, Mercedes Rizo-Baeza4
1 Departament of Research, Biopartner SL. Alcoy, Alicante, Spain.
2 Department of Pharmacology, Pediatrics and Organic Chemistry, Miguel Hernández University, San Juan de Alicante, Alicante, Spain.
3 Department of Clinical Medicine, Miguel Hernández University, San Juan de Alicante, Alicante, Spain
4 Department of Nursing, University of Alicante, San Vicente del Raspeig, Alicante, Spain.
* Autor para correspondencia.
Torró Montell L, Cortés-Castell E, Veciana-Galindo C, Sirvent-Segura E, Gil-Guillén F, Rizo- Baeza M. Influencia de los polifenoles del olivo en los niveles de glucosa y colesterol en el pez medaka. JONNPR. 2020;5(5):478-90. DOI: 10.19230/jonnpr.3356
![]()
This work is licensed
under a Creative
Commons
Attribution-NonCommercial-ShareAlike 4.0 International License
La revista no cobra tasas por el envío de trabajos,
ni tampoco cuotas por la publicación de sus artículos.
Abstract
Background. Polyphenol-rich olive extracts are non-toxic and have anti-inflammatory, neuroprotective and antiadipogenic effects in cell and animal models.
Objective. To evaluate the potential influence of olive extracts on the mechanisms of digestion and absorption of polysaccharides and fats by quantifying amylase, glucose, phospholipase, and cholesterol in the medaka fish model.
Material and methods. For each assay, six adult fish were placed in a tank with an extract (0.01% concentration), performing three replicates per extract. A control group with standard feeding was used. The same procedure was followed to study glucose, adding a polysaccharide-rich diet and a corresponding overfed control. The fish were maintained under these conditions for five days. Five olive extracts were used without attempting to purify the polyphenols due to possible synergistic effects. Total concentrations were between 2-116mg/g (mainly oleuropein and hydroxytyrosol). On completion, amylase, phospholipase A2, glucose and cholesterol were quantified in each group. All assays were conducted in triplicate. Enzyme activities were also studied in juveniles. Non-parametric tests were used to determine possible differences, considering p<0.05 to denote statistical significance.
Results. Polyphenol extracts were not toxic at a concentration of 0.1%, ten times higher than the concentration used. An overall decrease in glucose levels was observed in fish overfed with carbohydrates with the addition of the extracts, but without returning to the levels in the control group with standard feeding (between 15-40% decrease). There was no impact on amylase in adults or juveniles, an overall but not significant decrease in cholesterol, and an overall increase in phospholipase in the juveniles.
Conclusion. Olive extracts rich in polyphenols lower glucose levels in overfed fish.
Keywords
Polyphenols; olive; glucose levels; cholesterol levels; medaka fish
Resumen
Antecedentes. Los extractos de aceitunas ricos en polifenoles no son tóxicos y tienen efectos antiinflamatorios, neuroprotectores y antiadipogénicos en modelos celulares y animales.
Objetivo. Evaluar la influencia potencial de los extractos de aceituna en los mecanismos de digestión y absorción de polisacáridos y grasas mediante la cuantificación de amilasa, glucosa, fosfolipasa y colesterol en el modelo de pez medaka.
Material y métodos. Para cada ensayo, se colocaron seis peces adultos en un tanque con un extracto (al 0,01%), realizando tres repeticiones por extracto. Se usó un grupo control con alimentación estándar. Se siguió el mismo procedimiento para estudiar la glucosa, agregando una dieta rica en polisacáridos y un control de sobrealimentados. Los peces se mantuvieron en estas condiciones durante cinco días. Se usaron cinco extractos del olivo sin intentar purificar los polifenoles debido a posibles efectos sinérgicos. Las concentraciones totales fueron entre 2-116 mg/g (principalmente oleuropeína e hidroxitirosol). Al finalizar, se cuantificaron amilasa, fosfolipasa A2, glucosa y colesterol en cada grupo. Todos los ensayos se realizaron por triplicado. Las actividades enzimáticas también se estudiaron en alevines. Se utilizaron pruebas no paramétricas para determinar posibles diferencias, considerando p <0.05 para significación estadística.
Resultados. Los extractos de polifenoles no fueron tóxicos a una concentración de 0.1%, diez veces mayor que la concentración utilizada. Se observó una disminución general en los niveles de glucosa en peces sobrealimentados con carbohidratos con la adición de extractos, pero sin volver a los niveles del grupo control con alimentación estándar (disminución entre 15-40%). No hubo impacto sobre la amilasa en adultos o juveniles, se observó una disminución general pero no significativa del colesterol y un aumento general de la fosfolipasa en los juveniles.
Conclusión. Los extractos de aceitunas ricos en polifenoles reducen los niveles de glucosa en peces sobrealimentados.
Palabras clave
Polifenoles; olivo; niveles de glucosa; niveles de colesterol; pez medaka
Introduction
Food metabolism is very complex and is mediated by many enzymes and transporters that transform food into the nutrients to be assimilated by the body. For this, food must be ingested, digested, absorbed and transported to the target cells, where it is either degraded or stored. Among the energy nutrients are carbohydrates and fats that, to be assimilated by the body, need the help of enzymes to convert carbohydrates into glucose and fats into fatty acids. Many digestive amylases and lipases have been described that hydrolyze starch into maltose and ester bonds into free fatty acids, respectively, in the intestine. Inhibition of these enzymes or of the intestinal absorption mechanisms of simple biomolecules may help to reduce blood levels of these enzymes.
In addition to their nutrient delivery functions, many foods have the ability to provide health benefits and reduce the risk of disease. These are called functional foods. The benefits reported include the reduction of cholesterol and triglyceride levels and the control of blood glucose levels. The PREDIMET study concluded that a diet enriched with extra virgin olive oil significantly reduces cardiovascular risk (1). In addition, the ESCARVAL study found that the most important protective factor against cardiovascular disease is the increase in HDL, and this fraction of cholesterol increases in populations with greater adherence to the Mediterranean Diet (2) .
Foods rich in polyphenols have shown health benefits. Polyphenols, specifically, have been shown to have beneficial effects in obese patients, either through their anti-inflammatory action (3) or by reducing fat deposits, as has been shown in rats (4). Other studies have found that dietary polyphenol supplementation is associated with reduced obesity (5,6) and that these effects occur by inducing or suppressing genes associated with adipogenesis, lipolysis, and fatty acid oxidation (7-9).
The European Food Safety Authority has stated that olive polyphenols have protective effects on the cholesterol profile, an antihypertensive effect, and anti-inflammatory properties, among other health benefits, and have therefore been granted a health claim (10) the use of which is regulated by European Regulation 432 of 2012 (11,12).
In a previous study conducted by our group, using polyphenol-rich extracts from olive processing materials, we observed that these extracts are not toxic in cell and fish models (12). These extracts are also shown to have anti-inflammatory (13), neuroprotective (14), and antiadipogenic effects in mouse fibroblasts with a reduction in fat deposits and in the number of adipocytes in cell differentiation, accompanied by a decrease in the expression of leptin and PPARγ genes (15).
The objectives of this paper were to evaluate the potential influence of polyphenol-rich olive extracts on the mechanisms of digestion and absorption of polysaccharides and fats by quantifying amylase and glucose levels and phospholipase activity and cholesterol levels, respectively, using the medaka fish (Oryzias latipes) model.
Material and Methods
Subjects
Medaka fish were used as a model because they offer great advantages over higher vertebrates, as well as having a genomic homology with humans of over 70%. Medaka provide a much faster and more economical model due to their high reproduction rate, rapid maturation and the lower cost of feeding and maintaining the fish (24).
Extracts used
Five extracts were used, obtained from different components involved in the processing of table olives (raw materials from olive groves). To prepare these extracts, the raw materials were subjected to different extraction techniques with different solvents, after which the solvents were concentrated and separated so that no traces of organic solvents remained. Because there are proven synergies between different phytochemicals, we did not attempt to purify any of the polyphenols present. The extracts were processed by freeze-drying to ensure their optimum preservation and use in different forms (Biopartner carried out a stability study of the extracts under these conditions ensuring their activity, measured as antioxidant power and total polyphenols, for at least two years). The main characteristics of each of the extracts are shown in Table 3.
Table 3. Characteristics of the extracts used.
|
|
Total Polyphenols |
Total antioxidants |
Oleuropein
|
Hydroxytyrosol |
|
Extract |
(mg/g extract) |
(mmoles TPTZ/100g) |
(% w/w) |
(% w/w) |
|
E1 |
116 |
13 |
1.24 |
10.17 |
|
E2 |
49 |
5 |
1.27 |
1.99 |
|
E3 |
23 |
3 |
1.12 |
0.20 |
|
E4 |
68 |
8 |
0.50 |
0.03 |
|
E5 |
7 |
2 |
<0.003(DL) |
<0.001(DL) |
TPTZ, 2,4,6-tri(2-pyridyl)-1,3,5-triazine; DL, detection limit.
Variables analyzed
Amylase activity, glucose levels, phospholipase A2 activity and cholesterol levels were evaluated.
The procedures used were as follows:
Toxicity study. To determine the potential toxicity and acceptable levels of the different polyphenol extracts used, the potential toxicity in juvenile medaka was measured at different extract concentrations (0.1%, 0.5%, and 1%) at 5, 24 and 48 hours of incubation. Six embryos per well were placed in 96-well plates with Yamamoto medium (Sigma-Aldrich). Each assay was performed in triplicate, scoring the mortality of the juveniles with four points at 5 hours, two points at 24 hours and one point at 48 hours and scoring impaired movements as two points if observed at 5 hours, one point at 24 hours and 0.5 point at 48 hours. The mean score was determined for each extract and for the controls.
Quantification of glucose and cholesterol. For each assay, six adult fish (each extract, control, and overfed control) were placed in tanks to which we added the extract at a water concentration of 0.01%. and a carbohydrate-rich diet (Renfrewshier, UK). Three replicates were performed for each extract tested. After five days of exposure to the extract, in the fish used to quantify glucose a main vein was sectioned and blood glucose levels were measured. At the same time, controls were carried out under standard feeding conditions and standard feeding plus overfeeding with carbohydrates, both without extract supplementation. Glucose was quantified using blood glucose test strips (Sigma-Aldrich) following the method described by Zang (25,26).
The fish used to quantify cholesterol were frozen in liquid N2 and maintained at -40ºC until analysis. Before thawing, the fish were ground using a glass mortar and pestle and cholesterol was quantified in the supernatant using the method previously described for zebrafish (27).
Quantification of amylase and phospholipase A2 enzyme activity. Quantification was conducted with three- to four-day-old juveniles and also with adult fish. Three juveniles were placed in each well, adding 0.01% dissolution of the extract to the Yamamoto control medium. The same procedure was performed with the adult fish but in tanks. After 24 hours of exposure, 5.28 μl of DQStarch (50μM) amylase substrate solution (EnzChek® Ultra Amylase Assay Kit, Thermo Fisher) was added for amylase determination by incubating for 1 hour. To quantify phospholipase A2, after 24 hours of exposure to the extracts, we added 5.28μl of PED6 solution (50 μM) ((N-((6-(2,4-Dinitrophenyl)amino)hexanoyl)-2-(4,4-Difluoro-5,7-Dimethyl-4-Bora-3a,4a-Diaza-s-Indacene-3-Pentanoyl)-1-Hexadecanoyl-sn-Glycero-3-Phosphoethanolamine, Triethylammonium Salt) (Sigma-Aldrich), phospholipase substrate, incubating for 1 hour. In both cases, the juvenile fish were transferred to an Eppendorf tube and washed twice with Yamamoto solution. All supernatant liquid was removed and the fish were frozen with liquid N2. After adding 50 μl of distilled water to the tube, the samples were ground in a glass mortar. A further 200 μl of distilled water was added and the samples were centrifuged for 5 minutes at 14000 rpm (4ºC). Each well of a 96-well plate was filled with 100 μl of the supernatant and fluorescence was quantified using a Glomax Multi-Mode Detection System (with the Blue and AFC filters). Six assays were conducted in triplicate for each extract and untreated control in the juvenile fish and three assays in duplicate for the adult fish.
Statistical analysis
The variables were defined using means and standard deviations, normalized to the controls. To determine if there were differences and given that the variables did not have a normal distribution, non-parametric tests were used. The significance level was considered to be p<0.05.
Ethical considerations
The research was adjusted to the established legal and ethical procedures, carried out under the authorization of the Ministry of Agriculture, Environment, Climate Change and Rural Development of the Generalitat Valenciana (Spain) (ref. 2016 / VSC / PEA / 00123 type 2).
Results
Toxicity study
The control scored 0. In comparison, E1 scored 6, 5.33, and 2 at concentrations of 1%, 0.5%, and 0.1%, respectively. E2 scored 0.67, 0.22, and 0.06 at concentrations of 1%, 0.5%, and 0.1%, respectively. E3 only scored above 0, specifically 0.11, at 0.5% and E4 scored 0.28 at 0.1%. E5 presented no adverse events to any of the concentrations.
Study in adults
The values expressed in Table 1 show that, overall, in overfed fish polyphenol extracts E1, E3 and E4 significantly lowered glucose values (Fig 1). Only E4 appears to have had an influence on the significant increase in amylase action. No significant differences were found in cholesterol, although it clearly decreased with all extracts by almost half, not reaching significance due to the large dispersion of cholesterol values in the control group. In phospholipase, a significant decrease was observed only in E4.
Table 1. Glucose, amylase, cholesterol and phospholipase values in adult medaka fish. Glucose and cholesterol values are referenced to the concentration in the controls and amylase and phospholipase values are referenced to the fluorescence in the controls at 510-570 nm.
|
Assay |
Glucose Mean ± SD |
p value |
Amylase Mean ± SD |
p value |
Cholesterol Mean ± DS |
p value |
Phospholipase Mean ± SD |
p value |
|
|
|
|
|
|
|
|
|
|
|
Control |
0.30±0.11 |
0.003 |
0.89±0.20 |
_ |
1.00±0.94 |
_ |
1.01±0.11 |
_ |
|
Overfed control |
1.00±0.24 |
_ |
NA |
_ |
NA |
_ |
NA |
_ |
|
E1 |
0.62±0.24 |
0.009 |
1.30±0.82 |
1.000 |
0.50±0.06 |
0.855 |
0.93±0.32 |
0.325 |
|
E2 |
0.78±0.55 |
0.197 |
0.89±0.34 |
0.806 |
0.36±0.11 |
0.150 |
0.94±0.50 |
0.453 |
|
E3 |
0.60±0.12 |
0.012 |
1.08±0.38 |
0.806 |
0.42±0.10 |
0.423 |
1.65±0.99 |
1.000 |
|
E4 |
0.59±0.25 |
0.019 |
1.44±0.43 |
0.019 |
0.54±0.09 |
0.631 |
0.70±0.12 |
0.010 |
|
E5 |
0.85±0.26 |
0.294 |
1.03±0.22 |
0.327 |
0.51±0.09 |
0.749 |
0.99±0.43 |
0.714 |
|
p-Kruskal-Wallis |
0.004 |
|
0.410 |
|
0.089 |
|
0.297 |
|
NA, not applicable
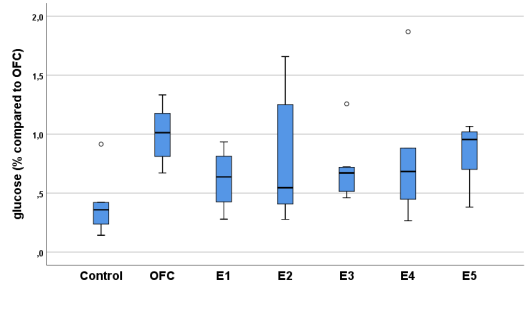
Figure 1. Relative glucose concentrations in controls and in overfed adult fish in the presence of extracts.
OFC, Overfed Control.
Study in juveniles
To confirm or reject an absence of influence of the extracts on enzymatic activity, the same experiment was performed on juveniles. The results, expressed in Table 2, show no influence on amylase with any of the extracts, while phospholipase increased significantly with all extracts except E1 (Fig 2).
Table 2. Relative values of amylase and phospholipase in juvenile medaka fish.
|
|
Amylase |
|
Phospholipase |
|
|
Assay |
Mean ± SD |
p value |
Mean ± SD |
p value |
|
Control |
0.95±0.32 |
_ |
0.95±0.14 |
_ |
|
E1 |
0.89±0.38 |
0.756 |
1.05±0.17 |
0.242 |
|
E2 |
1.17±0.51 |
0.195 |
1.20±0.20 |
0.014 |
|
E3 |
1.11±0.50 |
0.370 |
1.30±0.24 |
0.013 |
|
E4 |
0.84±0.40 |
0.334 |
1.68±0.67 |
0.001 |
|
E5 |
1.06±0.38 |
0.368 |
1.90±0.97 |
0.015 |
|
p-Kruskal-Wallis |
0.251 |
|
0.003 |
|
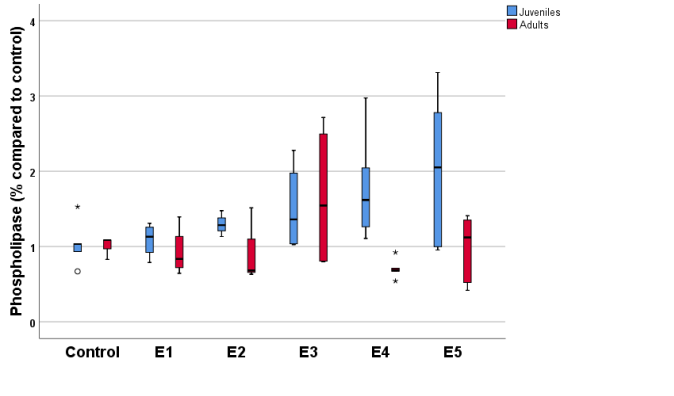 Figure
2. Relative activity of phospholipase in adults and juvenile
medaka fish in the presence of the different extracts analyzed.
Figure
2. Relative activity of phospholipase in adults and juvenile
medaka fish in the presence of the different extracts analyzed.
Discussion
Summary
Overall, the polyphenol extracts did not present an appreciable toxicity in juvenile fish at concentrations of 0.1%, which are ten times higher than those used for the main purpose of this study. Only E1 presented a small degree of toxicity. This has been verified and endorsed by EFSA (10) for polyphenols and specifically for polyphenol-rich olive extracts by our team (12). With the extracts there was a general decrease in glucose levels in overfed fish, with no impact on amylase in either adults or juveniles. There was a general but not significant decrease in cholesterol, and a general increase in phospholipase in juveniles.
Strengths and limitations
Inter-individual variability should be noted as a limitation, as it results in great variation between individuals. With a wide distribution of normal values, the number of trials must be increased as it is not possible to compare before and after exposure. For example, values for cholesterol, which is clearly lowered by the extracts, did not reach statistical significance because the control values were widely dispersed. Another limitation is that these results cannot be generalized to humans. Therefore, these data need to be verified through further animal studies and subsequently in humans.
One of the strengths within the complexity of the assays is that it is much easier and more economical to initiate studies of this type with fish due to their shorter life cycle duration, high reproduction, and lower cost compared to other assay methods, and they are also recognized as a suitable model in the study of obesity (16).
Comparison with the literature
Polyphenols from fruits and vegetables and present in red wine, green and black tea, coffee, chocolate, olive leaves, extra virgin olive oil and olives have biological effects including antioxidant, anticarcinogenic, and anti-inflammatory properties (17). There is also evidence of beneficial effects on obesity by inhibiting lipid accumulation (3), or by increasing fat oxidation and thermogenic effect (18). Synergistic antiadipogenic effects with other phytochemicals have also been reported (19). Thus, resveratrol and its metabolites exert a lipid-lowering function by reducing PPAR and lipoprotein lipase expression (20). Grape skin polyphenols suppress lipogenic enzyme activity and induce lipogenesis and oxidation in adipose tissue and mouse liver (21), while apple skin polyphenols prevent adiposity and increase inhibition of hypertrophic adipocytes (5). In addition, oleuropein reduces fat accumulation in 3T3-L1 cells during preadipocyte differentiation (22). A previous study by our group using mouse fibroblasts grown in the presence of polyphenols extracted from olive pits demonstrated reduced differentiation to adipocytes and less fat accumulation (15). The present work showed a decrease in glucose with the extracts, similar to that reported by other authors (23), confirming the decrease in cholesterol and the increase in phospholipase that assists in the mobilization and degradation of fats.
Implications for research
These data support the need for further study of the possible mechanisms for these effects, including the study of the possible activation or repression of genes involved in energy metabolism and studies in mammals to obtain evidence for their possible use as nutritional supplements in humans.
Conclusions
Extracts obtained from processing materials from olive groves rich in polyphenols, particularly, oleuropein and hydroxytyrosol, reduce glucose levels in overfed fish, as well as lowering cholesterol levels and increasing phospholipase A2 activity.
Funding
The line of research "Desarrollo y validación de alimentos saciantes mediante la integración de las señales pre y postingestivas" is supported by "Centro para el Desarrollo Tecnológico Industrial. Ministerio de Economía y Competividad". Spain (2015; ID-20150582).
Acnowledgements
The authors thank Maria Repice and Ian Johnstone for their help with the English version of the text.
Conflicts of Interest
None of the authors have potential conflicts of interest to be disclosed
Referencias
1. Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013, 368, 1279-1290.
2. Gil-Guillen, V.; Orozco-Beltran, D.; Redon, J.; Pita-Fernandez, S.; Navarro-Pérez, J.; Pallares, V. et al. Rationale and methods of the cardiometabolic Valencian study (Escarval-Risk) for validation of risk scales in Mediterranean patients with hypertension, diabetes or dyslipidemia. BMC Public Health. 2010, 10, 717.
3. Hsu, C.L.; Yen, G.C. Phenolic compounds: evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res. 2008, 52, 53-61.
4. Tian, C.; Ye, X.; Zhang, R.; Long, J.; Ren, W.; Ding, S. et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARγ-adiponectin pathway. PLoS One. 2013, 8, e53796.
5. Boqué, N.; Campión, J.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; San Román, B. et al. Screening of polyphenolic plant extracts for anti-obesity properties in Wistar rats. J Sci Food Agric. 2013, 93, 1226-1232.
6. Panickar, K.S. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol Nutr Food Res. 2013, 57, 34-47.
7. Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Bañuelos, O. et al. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res. 2013, 57, 1473-1478.
8. Manna, C.; D'Angelo, S.; Migliardi, V.; Loffredi, E.; Mazzoni, O.; Morrica, P. et al. Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J Agric Food Chem. 2002, 50, 6521-6526.
9. Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z. et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J Nutr Biochem. 2010, 21, 634-644.
10. EFSA. Panel on Dietetic Products, Nutrition and Allergies (NDA)2, 3. European Food Safety Authority (EFSA), Parma, Italy. EFSA Journal 2011, 9, 2033.
11. Reglamento (UE) Nº 432/2012 de la Comisión de 16 de mayo de 2012. DO L 136 de 25.5. 2012, p. 1.
12. Veciana, C.; Cortés, E.; Torro, L.; Sirvent, E.; Rizo, M.M.; Gil, V. Evaluación de la citotoxicidad y bioseguridad de un extracto de polifenoles de huesos de aceitunas. Nutr Hosp. 2014, 29, 1388-1393.
13. Cortés, E.; Veciana, C.; Torro, L.; Sirvent, E.; Rizo, M.M.; Gil, V. Anti-inflammatory activity of olive seed polyphenolic extract in the THP1-XBlue-CD14 Human monocytes cell line. Nutr Hosp. 2014, 30, 113-117.
14. Cortés, E.; Veciana, C.; Torro, L.; Sirvent, E.; Rizo, M.M.; Gil, V. Efecto sobre el neurodesarrollo y neuroprotección en pez cebra de un extracto polifenólico de huesos de aceituna. Nutr Hosp. 2014, 30, 338-342.
15. Veciana-Galindo, C.; Cortés-Castell, E.; Torró-Montell, L.; Palazón-Bru, A.; Sirvent-Segura, E.; Rizo-Baeza, M.M.; Gil-Guillén, V.F. Anti-adipogenic activity of an olive seed extract in mouse fibroblasts. Nutr Hosp. 2015, 31, 2747-2751.
16. Ichimura, K.; Kawashima, Y.; Nakamura, T.; Powell, R.; Hidoh, Y.; Terai, S. et al. Medaka fish, Oryzias latipes, as a model for human obesity-related glomerulopathy. Biochem Biophys Res Commun. 2013, 431, 712-717.
17. Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004, 79, 727-747.
18. Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006, 50, 176-187.
19. Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Synergism between resveratrol and other phytochemicals: implications for obesity and osteoporosis. Mol Nutr Food Res. 2011, 55, 1177-1185.
20. Lasa, A.; Churruca, I.; Eseberri, I.; Andrés-Lacueva, C.; Portillo, M.P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol Nutr Food Res. 2012, 56, 1559-1568.
21. Park, H.J.; Jung, U.J.; Lee, M.K.; Cho, S.J.; Jung, H.K.; Hong, J.H. et al. Modulation of lipid metabolism by polyphenol-rich grape skin extract improves liver steatosis and adiposity in high fat fed mice. Mol Nutr Food Res. 2013, 57, 360-364.
22. Kuem, N.; Song, S.J.; Yu, R.; Yun, J.W.; Park, T. Oleuropein attenuates visceral adiposity in high-fat diet-induced obese mice through the modulation of WNT10b- and galanin-mediated signalings. Mol Nutr Food Res. 2014, 58, 2166-2176.
23. Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol Nutr Food Res. 2013, 57, 48-57.
24. Schartl, M.; Kneitz, S.; Wilde, B.; Wagner, T.; Henkel, C.V.; Spaink, H.P. et al. Conserved expression signatures between medaka and human pigment cell tumors. PLoS One. 2012, 7, e37880.
25. Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish. 2013, 10, 425-432.
26. Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. Repeated Blood Collection for Blood Tests in Adult Zebrafish. J Vis Exp. 2015, 102, e53272.
27. Chu, C.Y.; Chen, C.F.; Rajendran, R.S.; Shen, C.N.; Chen, T.H.; Yen, C.C. et al. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS One. 2012, 7, e36474.
