REVIEW
Carcinomatous meningitis
Meningitis carcinomatosa
Maria Ajenjo González1, Naiara Cubelos Fernández2
1 Médico de Atención Primaria. Centro de Salud San Andrés del Rabanedo, León. España
2 Médico de Atención Primaria. Centro de Salud José Aguado, León. España
* Autor para correspondencia.
![]()
This work is licensed
under a Creative
Commons
Attribution-NonCommercial-ShareAlike 4.0 International License
La revista no cobra tasas por el envío de trabajos,
Abstract
Considered as the culmination of metastatic disease, although not exclusive to this phase, it is a common clinical-pathological complication caused by the dissemination of tumour cells in the meningeal membranes and cerebrospinal fluid. The dissemination takes place through the arachnoid vessels and by contiguity from the cerebral parenchyma and through the cerebrospinal fluid, the cells extend in sheet form all over the surface of the central nervous system, where they can also be grouped into nodules. The areas of preference of deposit are the basal cisterns, Silvio fissure, the hippocampus region and the lumbar region. It manifests generally with a meningeal and intracranial hypertension. It implies a short life expectancy, around 3-6 months in patients that receive chemotherapy. It is an incurable disease in spite of an intensive treatment. Patients with leukaemia, lymphoma and breast cancer are those that present a greater response to treatment and a longer life expectancy, around one year1. Occasionally, carcinomatous meningitis represents the first clinical evidence of cancer (in myeloblastic leukaemia) and should be included in the differential diagnosis in patients with subacute or chronic neurological manifestations, even when limited to a single level of neuroaxis.
This disease is a major oncological problem because it is becoming more frequent due to the increase in survival in certain cancers in which, a control of the disease can be achieved outside the neuroaxis and it is precisely in the central nervous system (CNS) where many of the drugs with antitumor activity do not penetrate due to the presence of a blood-brain barrier, which determines that the CNS is a "sanctuary" to which these drugs do not have access(2).
An early diagnosis through cerebrospinal fluid (CSF) as well as an early treatment of the disease may offer the best possibility of symptomatic control and prevention of the establishment of irreversible neurological deficits that compromise the patient's quality of life to a great extent, also an earlier treatment might prolong their survival. In spite of this, due to the few and nonspecific symptoms present in the patients and the low sensitivity and specificity of the information obtained in the biochemical study of CSF, considered "gold standard" the diagnosis is a challenge. With this method, negative result is present in more than 45% of the samples. At the present, specific monoclonal antibodies against identified non-haematological malignancies are increasing, and this information is especially useful to know the cellular lineage and to diagnose micrometastasis.
Keywords
Carcinomatous meningitis; Headache; Neurological symptoms in the patient with cancer
Resumen
Considerada como la culminación de la enfermedad metastásica, la meningitis carcinomatosa (MC), aunque no es exclusiva de esta fase, supone una complicación común clínico-patológica originada por la diseminación de células tumorales en la leptomeninge y líquido cefalorraquídeo (LCR). La diseminación tiene lugar a través de los vasos aracnoideos y por contigüidad desde el parénquima cerebral; a través del líquido cefalorraquídeo. Las células se extienden en forma de sábana por toda la superficie del sistema nervioso central, donde también pueden agruparse formando nódulos. Las zonas de preferencia de depósito son las cisternas basales, cisura de Silvio, región hipocámpica y la cola de caballo. Clínicamente se manifiesta con el desarrollo de un cuadro meníngeo y de hipertensión intracraneal. Implica una corta esperanza de vida, en torno a 3- 6 meses. Se trata de una complicación incurable a pesar del tratamiento intratecal, de quimioterapia sistémica y radioterapia. Los pacientes con leucemia, linfoma y cáncer de mama son los que presentan una mayor respuesta al tratamiento y por tanto una mayor supervivencia que puede ser en torno a un año (1). En ocasiones, la MC representa la primera evidencia clínica de cáncer (en la leucemia aguda linfoblástica) y debe incluirse en el diagnóstico diferencial en pacientes con manifestaciones neurológicas subagudas o crónicas, incluso cuando se halle limitado a un solo nivel del neuroeje.
Esta enfermedad supone un gran problema oncológico ya que cada vez es más frecuente debido al aumento de la supervivencia en determinados cánceres en los que puede conseguirse un control duradero de la enfermedad fuera del neuroeje y es precisamente en el sistema nervioso central (SNC) a donde no llegan muchas de las drogas con actividad antitumoral por la presencia de una barrera hematoencefáica, que determina que el SNC sea un “santuario” a donde no tienen acceso estas drogas (2).
El diagnóstico precoz mediante el análisis del LCR y la indicación temprana de tratamiento ofrece la mejor posibilidad de control sintomático y de prevención del establecimiento de déficits irreversibles neurológicos que comprometen en gran medida la calidad de vida del paciente, pudiendo alargar en algunos casos, también su supervivencia. No obstante el diagnóstico puede ser un reto en las fases iniciales de esta complicación. Los síntomas pueden ser inespecíficos y los estudios radiológicos no concluyentes. Con cierta frecuencia el LCR no contiene células tumorales y tan solo sutiles cambios en la bioquímica del mismo. En determinadas neoplasias se pueden determinar antígenos tumorales que facilitan el diagnóstico.
Palabras clave
Meningitis carcinomatosa; Cefalea; Síntomas neurológicos en paciente con cancer
Epidemiology
CM is diagnosed in 5-8% of patients with cancer(3,4), and is present in 20% in autopsy studies in patients with solid tumours(5). It represents 55% of the total tumour affectation of the CNS(6).
The tumours with histology of adenocarcinoma are the most frequent in presenting CM. The cancers that most frequently infiltrate leptomeningeal are: the breast, small cell lung and melanoma, in this order(7). In the so-called tumours of unknown origin is between 1% and up to 7%(8). In brain tumours, it appears in 1- 2% of patients, being associated mainly with tumours of neuroectodermal origin, germinal tumours, and choroidal plexus(9).
Pulmonary and gastrointestinal(10) adenocarcinomas invade meninges through the spinal cord perivascularly, while squamous carcinomas have less potential for vascular invasion. The small lungs cell carcinomas, the breast ductal carcinomas and melanoma, have the greatest intensity of infiltration. The infiltration is made perivascular in the spinal nerves at early stages of the disease. The reason that metastases have a preference over certain organs can be explained anatomophysiologically by the blood circulation, lymphatic and other body fluids, by the proximity of tumours or metastatic locations with the subarachnoid space and through the nervous invasion.
The haematogenous route(11) is the most common route of invasion. The specificity of metastases to an organ, or the capacity to invade one, depends on a certain organ specific growth factors as well as a selective adhesion to the endothelium. Selective adhesion(12) is determined primarily by leukocytes, which travel through the endothelium expressing glycoproteins PSGL-1 and carbohydrates such as sialyl Lewys that are recognized by endothelium through E- and P-selectins and CD34. Subsequently, cytokines such as PAF, IL-8 or MPC-1 recognize leukocyte receptors and activate cell junctions with greater force that will serve as chemoattractant factors(13). Finally, integrins(14) such as Mac-1, LFA-1, VLA-4, bind to immunoglobulins present in endothelium(15), Ig-CAMS (ICAM-1, ICAM-2, VCAM-1). This process occurs inside and outside the tumours vessels, where the tumour cells express adhesion molecules, causing the distant dissemination or intraluminal thrombi, able to grow and extravasate by mechanical factors that cause endothelium disruption. Throughout the expression of VEGF, by certain tumours, angiogenesis is regulated and vascular permeability is increased, increasing this way, the potential for dissemination associating the presence of peritumoral edema. It is known that bacterial infections cause a great endothelial activation, helping the extravasation of the cancerous cells in blood(10). This form of invasion is characterized by the absence of direct effects of tumour cells on the blood vessels walls(11,12).
With the progression of the disease, a tumour is able to invade the spinal cord from the anterior medial fissure to the anterior horns through the central artery or in rarer cases directly penetrating the parenchyma, regardless of the vascular pathway after affecting the meninges. The invasion of the parenchyma causes circular necrosis around the white matter, a consequence of the zonal destruction of the vessels by tumour proliferation. Another form of spinal cord damage consists in the Wallerian degeneration of the posterior medullary horns. At the level of cauda equina, the damage occurs directly to the nerve fibres, a common characteristic of CM that involves periradicular infiltration to the centripetal endoneurium(13,14). This involves the loss of nerve fibres, a characteristic process of epidermoid carcinomas(15) and secondly by the Wallerian degeneration.The invasion of lymphatic vessels by tumour cells also causes medullary necrosis, where the tumour cells directly invade the lumen of the vessel causing thrombosis and vasculitis in some cases. Physiopathology
The CM occurs when tumour cells migrate from a primary site and infiltrate the meningeal membranes of the brain and spinal cord. The success of the dissemination to the subarachnoid space of primary tumour cells that do not affect the central nervous system (CNS) depends on local cellular interaction, microenvironment and induction of angiogenesis and of cell proliferation.
This specific tropism for CNS of determinate tumours involves the mentioned molecular mechanisms, genetic and epigenetic mechanisms and the existence of a "tumour microenvironment", defined as "Seed and Soil hypothesis" and described by Stephen Paget more than 100 years ago, which establishes the interaction between the primary tumour (seed) and the soil organ, which is fundamental for the evolution of metastatic disease, involving local factors such as vascularization, pH, immune cells derived from the host or certain cytokines in nesting and development of metastases(16).
The transformation of a tumour cell into a metastatic tumour involves genetic, and permanent changes, which determine the expression of molecules with actions that favour the initiation of metastasis. This behaviour is determined by a gene that synthesizes a protein called "twist" responsible for turning on or off other genes. The twist protein is very active in early embryonic development and suppressed or inactivated when tissues are formed and organized.
The metastatic cascade theory defines a complex series of cancer cell passages that involves abandoning of the tumour’s original site, beginning with the rupture of the tissue's natural boundaries, the basal lamina, by invasion of the extracellular matrix(17). It follows the intravasation, phenomenon by which the tumour cell is introduced in a blood vessel or lymphatic and proceeds to its circulation in the organism. Normally, metastatic cells invade the subarachnoid tissue by arterial route, following the anatomical distribution of this vessels, affecting this way the areas that they irrigate. A retrograde (venous) pathway of dissemination through the Batson venous plexus is also possible, more common in retroperitoneal, gastrointestinal or uterine tumours. The lymphatic dissemination does not affect CNS because it does not contain lymphatic vessels.

The hematoencephalic barrier(18), is a physical and chemical protection of the CNS, is formed by endothelial cells connected with strong intercellular junctions and the base of the astrocyte membrane, in its final part, (neurovascular junction) that presents a wide permeability as well as intra and intercellular signals control.
The tumour cells are able to migrate along this endothelial barrier and transendothelially through molecular interactions between endothelial and tumour cell receptors. The molecules that intervene are:
- Selectins: are carbohydrates expressed in endothelial cells, platelets and leukocytes. There are several types of selectins (P-selectins, CD62P, in platelets and endothelial cells, L-selectins, CD62L, present in leukocytes and E-selectin, CD62E, in activated endothelial cells). Selectin receptors, such as Sialyl-Lewis x, P-selectin glycoprotein ligand 1 (PSGL-1), E-selectin ligand 1 (ESL-1), CD44 and B2-integrins especially. These selectins, which also play a role in inflammation and immune response, are the fundamental step for leukocyte displacement. Cancer cells also express ligands of selectins and use leukocyte-like migration mechanisms; all this facilitates the endothelial adhesion and contributes to create a favourable tumour microenvironment. In addition, platelets bind to tumour cells by P-selectin recognized by immune cells(18).Integrins: are transmembrane heterodimeric glycoproteins that facilitate intercellular binding and extracellular matrix formation for cell adhesion and migration. They provide a greater capacity of tumour anchorage to the endothelium. The integrin alpha-v-3 beta favours the interaction between platelets and the formation of the tumour micro thrombus. Integrin beta-4 produces signals indirectly by inducing the VEGF-dependent ErbB 2 receptor in the brain vasculature in tumour cells. These findings imply new levels of antitumor treatment(19).Chemokines: are cytokines with chemoattractant function to guide the cells in their migration and mediates the specific tropism (the chemokines CXCR4 and CXCL12 participate in the migration of breast tumour cells and CXCR4 also participates in the migration of tumour-derived cells of small cell lung). These findings serve to investigate the development of preventive drugs for the development of neurological manifestations of dissemination.Other factors, such as cyclooxygenase 2 (COX2) that favours cellular extravasation and increases vascular permeability under conditions of inflammation, epidermoid growth factor (EGFR) that has been implicated in growth and the capacity for cellular invasion, or the Rho kinase, a sign that has been implicated in intracellular binding and favours transendothelial migration are also activate pathways found in tumour dissemination and its antagonism is a step in the treatment of certain tumour types.
After having crossed the hematoencefalic barrier, tumour cells are found in a tumour microenvironment composed of cerebrospinal fluid, cerebral parenchyma and paracrine molecular signals(20).
Cerebrospinal fluid (CSF): It is an acellular medium that provides the means of transport to the tissues. It consists of water, proteins and polysaccharides, varying with age in the proportions presented by its components. The CSF forms the perineurium around the neuronal bodies and proximal dendrites. In order for the tumour to colonize CSF, the degradation of its components through molecules such as heparinases, endoglycoside (endoBglucuronidasa), which degrades heparan sulphate, the main growth component of CSF proteoglycans, and metalloproteases, which are zinc dependent endopeptidases which are involved in CSF degradation as well.
- The tumour interaction with the cerebral parenchyma: the cerebral parenchyma is composed mostly of astrocytes, which are involved in CM pathogenesis due to the ability to express CSF degrading heparinases, cytokines that contribute to the production of paracrine signals and the activation capacity of cell division. The microglia, the CNS's main immune system, is capable of activating inflammatory signals (in these lesions it is common to see gliosis in response to brain damage), neurodegeneration and the cancer itself by recruiting tumoral anuclear cells through the recognition of specific receptors and chemokines. This system produces macrophages that can be invaded by the tumour using several mechanisms previously mentioned.
Paracrine signs: includes the neurotropin and angiogenic factors. Neurotropins possess neuronal growth factors (NGFs) expressed in the CNS that stimulate the neurogenesis by inhibiting apoptosis, by promyotic factors and chemotactic properties; the tumours interact with different receptors. These neurotropins seems to stimulate cell invasion, growth and migration. Angiogenesis is a fundamental step in tumour formation, growth, and metastatic capacity.
Primary tumours can also be disseminated in the leptomeninges by direct contact or through the CSF. The risk of CM is higher after a surgical resection of a metastatic lesion in the posterior fossa due to the possible interruption of the metastatic pseudocapsule, which can cause tumour dissemination in CSF as well as by contact of the tumour with residual fragments during the surgery(21).
After the tumour has metastasized to the vertebral area, extension may occur along the nerve or along lymphatic pathways in the subarachnoid space, where the tumour spreads in the CSF and may invade the leptomeningeal tissues.
Clinical manifestations
The CM classically presents the involvement of three neurological domains: (a) involvement of the cerebral hemispheres (15%), (b) of the cranial nerves (35%), (c) and spinal cord and nerve roots (60%)(22). The most common manifestations of dysfunction of the cerebral hemispheres are headache and changes in mental state (along with optic neuropathy as a symptom of cranial nerve dysfunction, are the most commonly encountered signs). Confusion, cognitive impairment, isolation and hemiparesis are also included(23).The headache, is present in 30-40% of the CM patients(24) is usually the initial symptom. It is a non-specific, bifrontal, diffuse or located pain in the base of the skull that radiates to the neck. It is usually associated with dizziness, nausea and vomiting. Pain episodes are often intensified, with peaks coinciding with increases in intracranial pressure that may lead to loss of consciousness or visual disturbances such as myodesopsis. These pressure sensation tend to appear more frequently with postural changes, mainly standing, and are usually due to the presence of hydrocephalus, which causes apraxic gait, cognitive disorders and urinary incontinence. This increase in intracranial pressure occurs in the absence of true hydrocephalus, called pseudo-normocephaly, with falsely normal ventricles size due to compression of the inflamed cerebral parenchyma. Confusion and somnolence are present between 31- 52% may be the only manifestations of CM(25).
The manifestations due to cranial t up to 50%nerve dysfunction are the affectation of the VI cranial nerve, most frequently affected, followed by the III and IV pairs. These symptoms are present in 30% of patients(26) but have some anatomical damage(30). The involvement of trigeminal and cochlear nerves ( V and VIII pair) might cause motor or sensorial dysfunction. When the spinal cord is affected, the symptoms will depend of the level of this affectation.
The radicular pain as well as the progression to the neck, the back and the lower extremities. Neck stiffness is only present in 15% of cases. Faecal or urinary incontinence may occur and retention is possible in lower spinal lesions. The clinical examination signs often are different from the perceived by the patient(27).
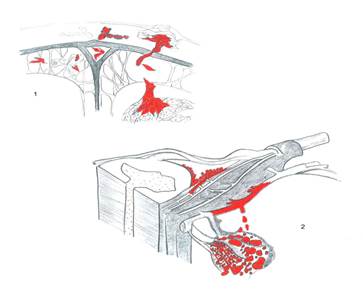
Image 1. Tumour invasion of the meninx.
Image 2. Representative diagram of the spinal cord and the vascular-nervous relationship with the tumour.
Pathophysiology of signs and symptoms
- Hydrocephalus and increased intracranial pressure: due to tumour infiltration of the brain, Silvio fissure and reactive inflammation and cerebral fibrosis, capable of reducing or blocking CSF flow, exerting pressure on the ventricles and thus increasing intracranial pressure. If the flow is interrupted near the sagittal groove or basal cisterns, the intracranial pressure will rise without obvious signs of cranial hypertension except for the increase in pressure present in a ventriculoperitoneal shunt(28).
Compression and invasion: focal neurological signs and symptoms occur due to the mass effect caused by the tumour, affecting mainly the 5th, 6th and 3rd cranial nerves due to the nasopharynx, prostate and lung carcinomas. Increased intracranial pressure and invasion of the parenchyma and spinal cord also might happen. Horner's syndrome is the result of paravertebral infiltration of the sympathetic nerves of the stellate ganglion and indicates a paraspinal and epidural spread of the tumour. Pancoast syndrome is a major involvement of the lower brachial plexus with hand atrophy, loss of sensation and weakness. The direct extension of an intraabdominal tumour may lead to lumbosacral plexopathy, such as disc herniation and meralgia, paresthetic pain, and weakness in the areas innervated by the affected nerves. At the cervical level, muscle function is not affected except for the loss of osteotendinous reflexes, which explains the predominance of symptoms in the lower limbs on the upper limbs(29).Metastatic infiltration in nerves causes axonal or Wallerian degeneration causing sensory neuropathies including pareesthesias and numbness of the hands and feet, a painful, incapacitating feeling, loss of postural and vibratory sensitivity, ataxia and unsteady gait. The loss of reflexes is a global affectation. Motor function may be acutely compromised, as is the case of Guillain-Barre syndrome with respiratory or bulbar symptoms due to nervous demyelination; in a subacute form, with distal predominance and associate with a great loss of weight, this cachexia being understood as a responsible part of the neuropathy, and chronically because of the chronic nervous inflammation that causes the plurineuronal demyelination. At the autonomic level, metastatic invasion causes sympathetic and parasympathetic symptoms due to the nuclear invasion of neurons causing dysautonomia such as orthostatic hypotension, pupillary alterations, impotence, precocious satiety, anorexia and constipation(30).
- Ischemia: it happens because of the compression of the local arteries located in the cerebral convexities or in the Virchow-Robin space, affecting the normal blood flow and neuronal oxygenation, causing stroke, epileptic seizures and a secondary encephalopathies due to global decline of blood flow.
- Metabolism: The diffuse encephalopathy present in these patients without apparent cause, could be caused by tumour cells, which compete with neurons for glucose and intermediate metabolites leaving the neurons with no necessary nutrients for their function.
Hematoencefalic barrier disruption: the rupture of this barrier as a result of direct tumour infiltration and the formation of new vessels, are another cause of altered CSF flow.
These neurological symptoms and signs were confirmed by a retrospective study done in the Dr Negrin hospital. Neurological symptoms and signs collected in a retrospective review of all patients admitted for suspected CM according to established criteria (epidemiology, clinical data, oncology, laboratory values, CSF parameters and neuroimaging techniques) at the University Hospital of Gran Canarias ( third level hospital that covers a sanitary area of approximately 450,000 inhabitants) are exposed in the table 1(31).
Diagnosis
The diagnosis of CM is difficult, and it is often necessary to repeat several tests until the diagnosis is reached. It must be considered that a high percentage of false negatives is present. Therefore, clinical signs and symptoms are essential for diagnosis. These clinical findings usually reflect the involvement of the brain, spinal cord, spinal roots and cranial nerves in the pathology. Often there are no association of symptoms. An MRI of the whole neuraxis and a lumbar puncture should be performed to obtain CSF for cell studies (CMF and cytology) and a biochemical test (glucose and protein concentration). The obtained CSF will be distributed in two tubes, one of them without adhesive agent, that will be sent for a cytology tests, and the other with an adhesive agent for a cytometry. The definitive diagnosis for all patients would be based on the detection of malignant cells in CSF along with radiological and biochemical compatible results. In general, cytological tests do not diagnose the particular tumour type.
Analysis of
cerebrospinal fluid
The most commonly used laboratory test for the diagnosis of CM is CSF analysis. An increase (> 200 mm H2O), in the opening pressure when puncture is done; a leukocyte increase (> 4leucos / mm3) an increased total protein (> 50 mg / dL), and a decrease in glucose (<60 mg / dL), is suggestive of CM, but not diagnostic. The basic rules for the cytological examination are the dilution of CSF, obtaining a suitable volume for the samples and their centrifugation. A microscopic examination with Giemsa staining and / or Papanicolau can be performed. Immunochemical analyses are performed to detect epithelial adhesion molecules (EpCAM).
Patients with positive CSF cytology represent a lower percentage in comparing with a negative one; the positivity increases when following cytological tests are performed, but the benefits of a second lumbar puncture are scarce. It has also been observed that levels of protein, glucose and malignant cells vary according to the site of CSF generation along the neuraxis. Even without obstruction in CSF flow; this finding reflects the multifocal nature of CM and explains why CSF tests obtained from one site distant from that of pathological areas. Thus, in the presence of spinal signs or symptoms, it is more likely to obtain cytological positive results. When the samples cannot be obtained within the level of the neurological symptoms, it is more likely to obtain a false negative result.
Despite the best efforts to obtain a positive simple, a number of patients with CM will still have a negative result, making the diagnosis and treatment more difficult.
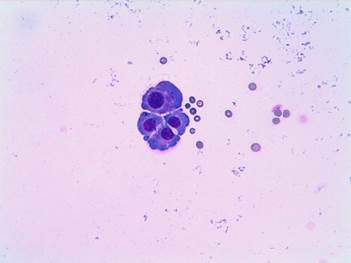
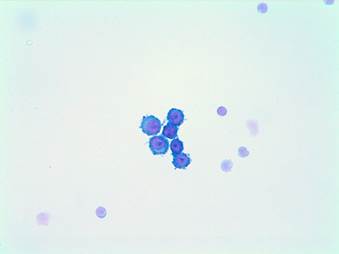
Image 3. ovarian cytology in cerebrospinal fluid. Cohesive group of epithelial habit cells with nuclear atypia: meningeal carcinomatosis.
The use of biochemical markers, immunohistochemically techniques and molecular biology on the CSF are aimed at detecting a biological marker of the disease. Numerous specific biochemical markers are detected, but have generally low sensitivity and specificity, except in the adenocarcinomas, the marker of which is the carcinoembryonic antigen (CEA), and extragonadal testicular and primary tumours related to alpha-fetoprotein (AFP) and beta-gonadotropin (B-hCG), whose serum levels have good specificity. Although not specific, other tumour markers, such as creatinine kinase-bb (CKBB), various isoenzymes, tissue polypeptide antigen (TPA), b2-microglobulin, b-glucuronidase (the most sensitive and specific in CM), lactate dehydrogenase isoenzyme-5 (LDH) and endothelial growth factor (VEGF) are strong indicators of CM, but are not cytologically sensitive for diagnosis. The use of immunohistochemically assays to detect monoclonal antibodies does not significantly increase diagnostic sensitivity on cytology alone. Determination of antibodies against specific cell membrane markers helps us to diagnose a leukaemia or lymphoma as the cause of this complication(32).Cytogenetic studies are often done with the aim of improving the diagnosis of CM. CMF and cellular DNA analysis by cytometry, cell chromosome analysis and fluorescence techniques detect numerical and structural anomalies in genes, signs of malignancy. These techniques provide us with additional diagnostic information, especially on haematological tumours, and are more sensitive than CSF cytology. The diagnosis is confirmed through PCR if the cytology is doubtful, which helps us to identify with a high probability the altered DNA fragments.In cases where there are no manifestations of systemic cancer and CSF examinations are inconclusive, a meningeal biopsy may be diagnostic, being more useful if a visible enhancements of contrast is observed during an MRI. Image testsSeveral diagnostic radiological methods have been evaluated for their diagnostic potential in both sensitivity and specificity. Techniques with intravenous MRI contrast, myelography and CT myelography were included. MRI images show changes in cerebrospinal fluid enhancement, and FLAIR signals and contrast-enhanced T1 sequences were attenuated; however, regardless of the technology used, the sensitivity remained relatively low.
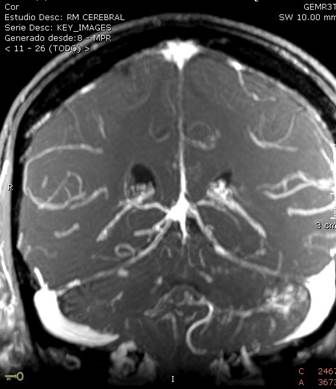
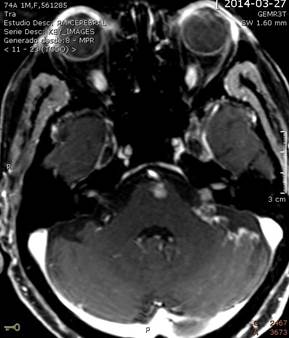
Image 4. Signs and symptoms of presentation of neoplastic meningitis.
II pair includes both decreased visual acuity and the presence of papilledema.Motor disorder includes hemiparesis, paraesthesia and tetraparesis.
Sensitive disorder includes clinical sensory facial.
Findings compatible with supra and infratentorial leptomeningeal dissemination predominantly in left folia. Lesion in the anterior region of the bulboprotuberancial junction, suggestive of nodular leptomeningeal metastasis of disseminated ovarian carcinoma.
The use of imaging tests is directed towards finding lesions along the CSF path and detecting alterations in the CSF flow. Currently, contrast MRI is considered superior in clinical practice than CT with contrast for the diagnosis of CM since it has greater sensitivity (with contrast and without contrast) to metastases and contrast uptake by leptomeninges.
The uptake of contrast by the leptomeninges is not a pathognomonic of CM, any irritation of the meninges will be observed in the images obtained, we must know that a banal irritation will be reflected in the MRI as a linear enhancement in that area. A lumbar puncture will rarely cause a prominence in the meninges, but it is preferable to perform the puncture after the imaging tests. It can also demonstrate parenchymal metastases (very common in melanoma) and subarachnoid nodules, generally in the lumbosacral or cauda equina region. Intracranial hypotension (especially after repeated lumbar punctures in these cases) also causes contrast uptake in the meninges.
Images that show enhanced cranial nerves or cranial or tentorial parenchyma if the tumour grows along the sulcus inducing neovascularization and also intra-extramedullary nodules in the spinal cord (especially in the equine cauda and Batsen plexus) in an MRI scan. Hydrocephalus can be considered diagnostic of CM in patients with cancer(36).
|
SYMPTOMS. |
% |
|
Sensitive disorder |
29.7 |
|
Alteration of mental state |
18.9 |
|
Diplopia |
32.4 |
|
Alteration of coordination |
10.8 |
|
Radicular or spinal pain |
8.1 |
|
Crisis |
5.4 |
|
|
|
|
SIGNS |
|
|
Alteration of mental state |
18.9 |
|
Meningism |
5.4 |
|
|
|
|
CRANIAL NERVES. |
|
|
II |
13.5 |
|
III |
10.8 |
|
IV |
2.7 |
|
V |
16.2 |
|
SAW |
18.9 |
|
VII |
2.7 |
|
VIII |
0 |
|
IX |
5.4 |
|
X |
8.1 |
|
XI |
0 |
|
XII |
2.7 |
|
Cerebellar Signs |
13.5 |
|
Motor Disorder |
27.0 |
Referencias
1. Fareeha Siddiqui, M. D., Lisa Marr, M. D., and David E. Weissman, M. D. Neoplastic Meningitis. Journal of Palliative Medicine. 2010. Vol. 12- 1.
2. Suki D, Khoury Abdulla R, Ding M, Khatua S, Sawaya R. Brain metastases in patients diagnosed with a solid primary cáncer. J Neurosurg. 2014.
3. Grisold W, Briani C, Vass A. Malignant cell infiltration in the peripheral nervous system. Handb Clin Neurol. 2013.
4. Nayak L, Fleisher M, González- Espinoza R, Lin O, Panageas K. Rare cell capture technology for the diagnosis of leptomeningeal metástasis in solid tumours. Neurology. 2013.
5. Du C, Hong R, Shi Y, Yu X, Wang J. Leptomeningeal metástasis from solid tumors.J Neurooncol. 2013.
6. Jiménez Mateos A, Cabrera Naranjo, González Hernández A., Fabre Pi O, Diaz Nicolás S., López Fernández JC. Neoplastic meningitis. Neurologia 2010. Vol. 26, pp 227- 32.
7. John Souglakos, Lambros Vamvakas, Stella Apostolaki, Maria Perraki, Zacharenia Saridaki, Irine Kazakou, et al. Central nervous system relapse in patients with breast cáncer in associated with advanced stages, with the presence of circulating occult tumour cells and with the HER2/neu status. 2010. Breast Cáncer Res.
8. N Nathoo, A. Chahlavi, G. H. Barnett, and S. A. Toms. Pathobiology of brain metastases. J. clin Pathol. 2008.
9. Beasley KD., Toms S. A. The molecular pathobiology of metástasis to the brain. Neurosurg Clin N. Am. 2011
10. Preusser M. Capper D., Iihan- Mutlu A. Berghoff A. S., Bimer P., Bartsch R., et al. Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathology. 2012.
11. Brian J Scott, Santosh Kesari. Leptomeningeal metastases in breast cáncer. Am J Cancer. 2013.3(2)
12. Yamaguchi Y, Ogawa M. Interaction between neutrophils and endotelial cells following ischemia/ reperfusion. 2000.
13. Kevin G Phillips, Peter Kuhn, Owen J. T. McCarty. Physical Biology in cáncer. The physical biology of circulating tumour cells. American Journal of Physiology. 2013.
14. Karen F. Chambers, Joanna F. Pearson, Naveed Aziz, Peter o´Toole, David Garrod, Shona H. Lang. Stroma regulates Increased Epithelial Lateral Cell Adhesion in 3D Culture: A Role for Actine/ Cadherin Dynamics. 2011.
15. Takey H, Rouah E, Barrios R. Intravascular carcinomatosis of central nervous system due to metastatic inflammatory breast cáncer. J Neuropathology 2015.
16. Rohan Ramakrishna, Robert Rostomily. Seed, soil and beyond: The basic biology of brain metástasis. Surg Neurol 2012.
17. K. A. Kovacs, B. Hegedus, I. Kenessey, J. Timar. Tumour type- specific and skin región- selective of human cancers: another example of the “seed and soil” hypothesis.
18. Jebali J. Jeanneau C, Bazaa A, Mathieu S, El Ayeb M, Luis J, et al. Selectins as adhesión molecules and potential therapeutic target. 2011.
19. Karen F. Chambers, Joanna F. Pearson, Naveed Aziz, Peter o´Toole, David Garrod, Shona H. Lang. Stroma regulates Increased Epithelial Lateral Cell Adhesion in 3D Culture: A Role for Actine/ Cadherin Dynamics. 2011.
20. Matthias Preusser, David Capper, Aysegul IIhan- Mutlu, Anna Sophie Berghoff, Peter Birner, Rupert Bartsch, et al. Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol. 2012.
21. Bruno MK, Raizer J. Leptomeningeal metastases from solid tumors (meningeal carcinomatosis). Cancer Treat Res. 2005.
22. Le Rhun E. Taillebert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surgical neurology international. 2013.
23. C. Krarup, C. Crone. Neurophysiological studies in malignant disesase with particular reference to involvement of peripheral nerves.
24. Kim HG, Im SA, Keam B, Kim YJ, Han SW, Kim TM, et al. Clinical outcome of central nervous system metastases from breast cáncer: differences in survival depending on systemic treatment. J Neurooncol. 2012.
25. Robert A. Nagourney, Robert Hedaya, Markku Linnoila, Philip S. Schein. Carcinoid carcinomatous meningitis.
26. Marc C. Chamberlain. Neoplastic meningitis. The oncologist. 2008.
27. Chamberlain M, Soffietti R, Raizer J, Ruda R, Brandsma D, Boogerd W. Leptomeningeal metástasis: a Response Assessment in Neuro- Oncology critical review of endpoints and response criteria of published randomized clinical trials.Neuro Oncol. 2014.
28. Jung TY, Chung Wk, Oh IJ. The prognostic sifnificance of hydrocephalus in leptomeningeal metastases. 2014.
29. Patrick Y. Wen. Leptomeningeal metastases: pathophysiology. Neuro- oncology.2012.
30. Morris D. Groves. Leptomeningeal Disease.
31. A. Jiménez Mateos, F. Cabrera Naranjo, A. González Hernández, O. Fabre Pi, S. Díaz Nicolás, J. C. López Fernández. Neoplastic meningitis. Review of a clinical series. Neurologia. 2011.
32. Liu J, Jia H, Yang Y, Dai W, Su X, Zhao G. cerebrospinal fluid cytology and clinical análisis of 34 cases with leptomeningeal carcinommatosis.2012.
33. Sung Jin Kang, MD, Kwang Soo Kim, Yoon Suk Ha, So Young, Jong Kuk Kim, Min Jeong Kim. Diagnostic Value of cerebrospinal fluid level of carcinoembryonic antigen in patients with leptomeningeal carcinomatous metástasis. J Clin Neurol. 2010.
34. Bigner SH, Johnston WW. The cytopathology of cerebrospinal fluid. II. Metastatic cáncer, meningeal carcinomatosis and primary central nervous system neoplasms.
35. Csako G. Chandra P. Bronchioloalveolar carcinoma presenting with meningeal carcinomatosis. Cytologic diagnosis in cerebrospinal fluid.
36. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2012.
